Tetralogy of Fallot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
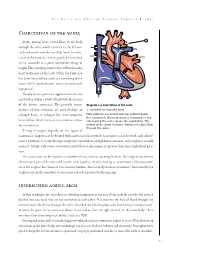
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

How to Recognize a Suspected Cardiac Defect in the Neonate
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcare- professionals/education/continuing-medical-nursing-education/neonatal- nursing-education-briefs/ Cardiac defects are commonly seen and are the leading cause of death in the neonate. Prompt suspicion and recognition of congenital heart defects can improve outcomes. An ECHO is not needed to make a diagnosis. Cardiac defects, congenital heart defects, NICU, cardiac assessment How to Recognize a Suspected Cardiac Defect in the Neonate Purpose and Goal: CNEP # 2092 • Understand the signs of congenital heart defects in the neonate. • Learn to recognize and detect heart defects in the neonate. None of the planners, faculty or content specialists has any conflict of interest or will be presenting any off-label product use. This presentation has no commercial support or sponsorship, nor is it co-sponsored. Requirements for successful completion: • Successfully complete the post-test • Complete the evaluation form Date • December 2018 – December 2020 Learning Objectives • Describe the risk factors for congenital heart defects. • Describe the clinical features of suspected heart defects. • Identify 2 approaches for recognizing congenital heart defects. Introduction • Congenital heart defects may be seen at birth • They are the most common congenital defect • They are the leading cause of neonatal death • Many neonates present with symptoms at birth • Some may present after discharge • Early recognition of CHD -

Congenital Cardiovascular Defects
Statistical Fact Sheet 2016 Update Congenital Cardiovascular Defects Congenital cardiovascular defects, also known as congenital heart defects, are structural problems that arise from abnormal formation of the heart or major blood vessels. ICD-9 lists 25 congenital heart defects codes, of which 21 designate specified anatomic or hemodynamic lesions. Defects range in severity from tiny pinholes between chambers that may resolve spontaneously to major malformations that can require multiple surgical procedures before school age and may result in death in utero, in infancy, or in childhood. The common complex defects include the following: Tetralogy of Fallot (TOF) Transposition of the great Trends in age-adjusted death rates attributable to arteries congenital heart defects, 1999 to 2013. Atrioventricular septal de- fects (ASD) Coarctation of the aorta Hypoplastic left heart syn- drome Incidence Congenital heart defects are serious and common condi- tions that have significant impact on morbidity, mortali- ty, and healthcare costs in children and adults. The most commonly report- ed incidence of congenital heart defects in the United States is between 4 and 10 per 1,000, clustering around 8 per 1,000 live births. Continental variations in birth prevalence have been reported, from 6.9 per 1000 births in Europe to 9.3 per 1000 in Asia. An estimated minimum of 40,000 infants are expected to be affected each year in the United States. Of these, about 25%, or 2.4 per 1,000 live births, require invasive treatment in the first year of life. Prevalence It is estimated that there are an estimated 650,000 to 1.3 million children and adults living with con- genital heart disease in the United States. -

Congenital Heart Disease Parent FAQ
Congenital Heart Disease Parent FAQ achd.stanfordchildrens.org | achd.stanfordhealthcare.org About Congenital Heart Disease What is congenital heart disease? Congenital heart disease, also called congenital heart defect (CHD), is a heart problem that a baby is born with. When the heart forms in the womb, it develops incorrectly and does not work properly, which changes how the blood flows through the heart. What causes congenital heart defects? In most cases, there is no clear cause. It can be linked to something out of the ordinary happening during gestation, including a viral infection or exposure to environmental factors. Or, it may be linked to a single gene defect or chromosome abnormalities. How common is CHD in the United States among children? Congenital heart defects are the most common birth defects in children in the United States. Approximately 1 in 100 babies are born with a heart defect. What are the most common types of congenital heart defects in children? In general, CHDs disrupt the flow of blood in the heart as it passes to the lungs or to the body. The most common congenital heart defects are abnormalities in the heart valves or a hole between the chambers of the heart (ventricles). Examples include ventricular septal defect (VSD), atrial septal defect (ASD), and bicuspid aortic valve. At the Betty Irene Moore Children’s Heart Center at Stanford Children’s Health, we are known across the nation and world for treating some of the most complex congenital heart defects with outstanding outcomes. Congenital Heart Disease Parent FAQ | 2 Is CHD preventable? In some cases, it could be preventable. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -

Prenatal Diagnosis, Associated Findings and Postnatal Outcome Of
J. Perinat. Med. 2019; 47(3): 354–364 Ingo Gottschalka,*, Judith S. Abela, Tina Menzel, Ulrike Herberg, Johannes Breuer, Ulrich Gembruch, Annegret Geipel, Konrad Brockmeier, Christoph Berg and Brigitte Strizek Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center https://doi.org/10.1515/jpm-2018-0316 anomalies, 30 (66.7%) had extracardiac anomalies and 13 Received September 18, 2018; accepted November 26, 2018; (28.9%) had chromosomal or syndromal anomalies. Due previously published online December 20, 2018 to their complex additional anomalies, five (11.1%) of our Abstract 45 fetuses had multiple malformations and were highly suspicious for non-chromosomal genetic syndromes, Objective: To assess the spectrum of associated anoma- although molecular diagnosis could not be provided. Dis- lies, the intrauterine course, postnatal outcome and orders of laterality occurred in 10 (22.2%) fetuses. There management of fetuses with double outlet right ventricle were 17 terminations of pregnancy (37.8%), two (4.4%) (DORV). intrauterine and seven (15.6%) postnatal deaths. Nineteen Methods: All cases of DORV diagnosed prenatally over a of 22 (86.4%) live-born children with an intention to treat period of 8 years were retrospectively collected in a single were alive at last follow-up. The mean follow-up among tertiary referral center. All additional prenatal findings survivors was 32 months (range, 2–72). Of 21 children who were assessed and correlated with the outcome. The accu- had already undergone postnatal surgery, eight (38.1%) racy of prenatal diagnosis was assessed. achieved biventricular repair and 13 (61.9%) received Results: Forty-six cases of DORV were diagnosed pre- univentricular palliation. -

Transcatheter Device Closure of Atrial Septal Defect in Dextrocardia with Situs Inversus Totalis
Case Report Nepalese Heart Journal 2019; Vol 16(1), 51-53 Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis Kiran Prasad Acharya1, Chandra Mani Adhikari1, Aarjan Khanal2, Sachin Dhungel1, Amrit Bogati1, Manish Shrestha3, Deewakar Sharma1 1 Department of Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal 2 Department of Internal Medicine, Kathmandu Medical College, Kathmandu,Nepal 3 Department of Pediatric Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal Corresponding Author: Chandra Mani Adhikari Department of Cardiology Shahid Gangalal National Heart Centre Kathmandu, Nepal Email: [email protected] Cite this article as: Acharya K P, Adhikari C M, Khanal A, et al. Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis. Nepalese Heart Journal 2019; Vol 16(1), 51-53 Received date: 17th February 2019 Accepted date: 16th April 2019 Abstract Only few cases of Device closure of atrial septal defect in dextrocardia with situs inversus totalis has been reported previously. We present a case of a 36 years old male, who had secundum type of atrial septal defect in dextrocardia with situs inversus totalis. ASD device closure was successfully done. However, we encountered few technical difficulties in this case which are discussed in this case review. Keywords: atrial septal defect; dextrocardia; transcatheter device closure, DOI: https://doi.org/10.3126/njh.v16i1.23901 Introduction There are only two case reported of closure of secundum An atrial septal defect (ASD) is an opening in the atrial ASD associated in patients with dextrocardia and situs inversus septum, excluding a patent foramen ovale.1 ASD is a common totalis. -

Double Outlet Right Ventricle (DORV)
Double outlet right ventricle (DORV) Vita Zīdere, MD FRCP Consultant Paediatric and Fetal Cardiologist • Complex lesion, represents 3 % of CHD seen in fetus • Both Great Arteries arise completely or predominantly from the morphological right ventricle • Associates with increased NT, genetic and extracardiac anomalies • Double outlet right ventricle is a spectrum of abnormalities • almost always has a VSD • heterogeneous with respect to size and position of VSD and relationship of GA • individual anatomy dictates surgical approach and outcome • It poses a number of challenges • Definition • Anatomic Variability (incl. normal or abnormal AV connections) • Surgical options DORV TOF type DORV “Simple” DORV Complex DORV Always VSD: Unbalanced Ventricles AVSD (often RAI or LAI) subaortic, MV atresia subpulmonary or uncommitted; Coarctation, IAA +/-PS Discordant AV connections Criss-cross heart Normal atrial situs Patent, concordant AV connections DORV Overriding aorta Both GA (parallel) from RV “50 % rule” Subpulmonary, subaortic or uncommitted VSD TOF type DORV Anatomical repair- Repair depends from GA relationship with VSD VSD closure & PS release LV to Ao baffle ASO with baffling VSD to neo-Ao Rarely BV repair is unfeasible L G Price 2004 G Price • True override is independent of septal axis • postnatally is assessed relative to chord of circle in short axis* *BR Wilcox, AC Cook, RH Anderson. Surgical anatomy of the heart,2004 Double Outlet Right ventricle v. Tetralogy of Fallot L L R R • Perimembranous VSD, overriding aorta • Pulmonary artery -

Tetralogy of Fallot.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Tetralogy of Fallot.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Tetralogy of Fallot Developed by Katie Girgulis, Dr. Andrew Mackie, and Dr. Karen Forbes for PedsCases.com. April 14, 2017 Introduction Hello, my name is Katie Girgulis and I am a medical student at the University of Alberta. This podcast was developed with the help of Dr. Andrew Mackie and Dr. Karen Forbes. Dr. Mackie is a pediatric cardiologist at the Stollery Children’s Hospital, and Dr. Forbes is a pediatrician and medical educator at the Stollery Children’s Hospital. This podcast is about the cardiac condition Tetralogy of Fallot (ToF). For teaching on the general approach to pediatric heart murmurs, please check out the ‘Evaluation of a Heart Murmur’ podcast on Pedscases.com. Slide 2 Learning Objectives By the end of this podcast, the learner will be able to: 1) Recognize the clinical presentations of ToF 2) Describe the four anatomical characteristics of ToF 3) Describe the pathophysiology of the murmur in ToF 4) Formulate initial steps when ToF is suspected 5) Delineate the treatment of hypercyanotic episodes 6) Summarize the definitive treatment for ToF Slide 3 Case – Baby Josh Let’s start with a clinical case: You are working with Dr. Smith, a family physician, during your family medicine rotation. Josh is a 4-month-old infant who is here for a well-baby check. -

Congenital Heart Disease: Recognition and Treatment Options – Anna Gelzer
CONGENITAL HEART DISEASE: RECOGNITION AND TREATMENT OPTIONS – ANNA GELZER Prevalence and Frequency Congenital heart disease in dogs: ≤ 1% (Canine clinic population of U Penn 1992) Cats estimated < 0.2% Prevalence likely underestimated (perinatal death, no murmur) Small % of cardiac disease overall, but most common in animals < 1 y old Common congenital cardiac defects in dogs (in the order of frequency): Subaortic stenosis (SAS) Pulmonic stenosis (PS) Patent ductus arteriosus (PDA) Tricuspid valve dysplasia (TVD) Ventricular septal defect (VSD) Tetralogy of Fallot (TOF) Persistent right aortic arch (PRAA) Atrial septal defects (ASD) Mitral valve dysplasia (MVD) Persistent left carnial venal cava Congenital cardiac defects in cats (in the order of frequency): Mitral valve dysplasia (MVD) Ventricular septal defect (VSD) Endocardial cushion defect (ASD+VSD) Patent ductus arteriosus (PDA) Aortic stenosis (SAS) Tetralogy of Fallot (TOF) Pulmonic stenosis (PS) Atrial septal defects (ASD) Tricuspid valve dysplasia (TVD) Physical exam finding in animals with most common congenital heart disease: The simple congenital heart defects are normally identified by the presence of a heart murmur. From the clinical examination, by localizing the heart murmur, identifying its radiation, assessing the precordial impulse and the peripheral pulse, a differential diagnosis list can be drawn up. The table summarizes the findings for some of the common defects identified in dogs and cats. Note, for these simple defects, mucus membrane color is normal (pink). -

Association of Hand Anomalies with Congenital Heart Lesions in Mentally Retarded, State- Institutionalized Patients Marian Grace Jordison Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1968 Association of hand anomalies with congenital heart lesions in mentally retarded, state- institutionalized patients Marian Grace Jordison Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Jordison, Marian Grace, "Association of hand anomalies with congenital heart lesions in mentally retarded, state-institutionalized patients" (1968). Yale Medicine Thesis Digital Library. 2756. http://elischolar.library.yale.edu/ymtdl/2756 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE MEDICAL LIBRARY 9002 01065 5752 seek ^ j IptxL-ffij y Li: Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/associationofhanOOjord ASSOCIATION OF HAND ANOMALIES WITH CONGENITAL HEART LESIONS IN MENTALLY RETARDED, STATE- INSTITUTIONALIZED PATIENTS Marian Grace Jordison B.A. Stanford University, 1964 A Thesis Submitted in Partial Fulfillment of the Requirement for the Degree of Doctor of Medicine Department of Epidemiology & Public Health Yale University School of Medicine April, 1968 y/3. TABLE OF CONTENTS PAGE Introduction. 1 Review of the literature. 3 I. Holt-Oram syndrome. 3 II. Epidemiology of congenital malformations. 6 A. Incidence. 6 B. -

Congenital Heart Defects
Congenital Heart Defects KNOW THE FACTS What is a congenital Detection heart defect? When can CHDs be detected? Congenital heart defects (CHDs) are problems present CHDs can be detected as early as the prenatal period or at birth that affect the structure or function of the heart. as late as adulthood (or escape detection altogether).2 They can affect how blood flows through the heart and The more severe the form of CHDs, the more likely it is to out to the rest of the body. be detected earlier.3 There are many types of heart defects, with different degrees of severity based on size, location, and other associated defects. Common examples include holes in different areas of the heart and narrow or leaky valves. In more severe forms of CHDs, blood vessels or heart chambers may be missing, poorly formed, or in the wrong place. Approximately 120 infant deaths are prevented each year with pulse oximetry screening What is the difference between congenital heart defect and congenital heart disease? These terms are often used interchangeably and How can CHDs be detected? are nearly synonymous. However, there is a slight There are a number of tools that can be used to aid difference between them. A congenital heart defect in the diagnosis of CHDs, including echocardiogram, refers specifically to a problem with the formation electrocardiogram, chest X-ray, chest CT, cardiac of the structure of the heart or major heart vessels in MRI, and prenatal ultrasound.4 One or more of these utero. Congenital heart disease refers to the clinical diagnostic tests may be ordered if a healthcare provider manifestation of an underlying anatomical defect, or finds a reason to suspect that the child has a CHD or if the more broadly describes functional problems which may child fails a newborn screening test.