NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -
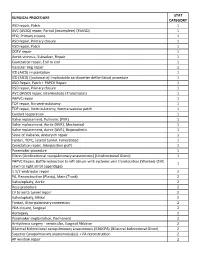
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

The Conversion to Rastelli's Type Operation from Patrick-Mcgoon's
General Thoracic and Cardiovascular Surgery (2019) 67:551–553 https://doi.org/10.1007/s11748-018-0942-x CASE REPORT The conversion to Rastelli’s type operation from Patrick-McGoon’s procedure of an adult with Taussig–Bing heart: a case report Keiichi Fujiwara1 · Kousuke Yoshizawa1 · Nobuhisa Ohno1 · Hisanori Sakazaki2 Received: 30 January 2018 / Accepted: 18 May 2018 / Published online: 11 June 2018 © The Japanese Association for Thoracic Surgery 2018 Abstract A 23-year-old female of Taussig–Bing heart with antero-posterior relation of the great arteries was underwent Patrick- McGoon’s intraventricular rerouting at 6 years old of age. The left ventricular outflow obstruction (peak pressure gradient of 100 mmHg) developed, and severe aortic valve regurgitation following bacterial endocarditis was noted. The conversion to Rastelli’s type operation and aortic valve replacement were performed successfully at 23 years old of age. She is doing well without any significant left or right ventricular outflow obstruction at 7 years postoperatively. Keywords Taussig–Bing heart · Intraventricular rerouting · Patrick-McGoon’s operation · Left ventricular outflow obstruction · Adult congenital heart disease Introduction Case Taussig–Bing heart is characterized by double-outlet right A 23-year-old female diagnosed of double outlet right ventricle with subpulmonary ventricular septal defect [1, 2]. ventricle with subpulmonary ventricular septal defect and Currently, the arterial switch procedure as anatomic repair, antero-posterior relation of the great arteries, was referred to connecting the left ventricle to aorta and the right ventricle our hospital for severe left ventricular outflow tract obstruc- to pulmonary artery, is a popular surgical management for tion following Patrick-McGoon’s intraventricular rerouting Taussig–Bing heart regardless of relation of the great arter- and moderate to severe aortic valve regurgitation following ies. -

Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon
r Me ula dic sc in a e V & f o S l u a Journal of Vascular r Nath et al., J Vasc Med Surg 2014, 2:2 g n r e u r y o DOI: 10.4172/2329-6925.1000135 J ISSN: 2329-6925 Medicine & Surgery Short Communication Open Access Severe Low Cardiac Output Following Pericardiectomy- Bird in Cage Phenomenon Mridu Paban Nath1*, Malavika Barman2 and Rajib Kr Bhattacharrya3 1Assistant Professor, Department of Anesthesiology & Critical Care, Gauhati Medical College Hospital, Assam, India 2Assistant Professor, Department of Biochemistry, Tezpur Medical College Hospital, Assam, India 3Professor & Head, Department of Anesthesiology & Critical Care, FAA Medical College Hospital, Assam, India A 28 year old boy was referred from a private hospital for evaluation long periods of myocardial compression contributing to remodelling of constrictive pericarditis. He was diagnosed for the same about 4 of the ventricles and to greater involvement of the myocardium in years back with history of worsening shortness of breath and fatigue. patients who have undergone long periods of symptomatic pericardial At the time of presentation, patient required supplemental Oxygen constriction, as in our patient with a history of 4 years of symptoms. and was New York Heart Association Class-IV heart failure. Physical MacCaughan et al. [4] have described haemodynamic abnormalities examination revealed distension of jugular veins with significant after pericardiectomy in the largest series available (231 patients). The ascites & hepatomegaly. Bilateral pedal edema was absent; however investigators noted a 28% incidence of LCOS postoperatively in their patient was on long term therapy with loop diuretics. About 1 litre of patients, with many of the perioperative deaths occurring in this low abdominal paracentesis was done to relieve tense ascites. -

Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Stra
Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Strawn, BSN, RN, CPN, Children’s Hospital & Medical Center, Omaha, Nebraska Jo Ann Nieves MSN, CPN, ARNP, PNP-BC, FAHA, Miami Children’s Hospital Bronwyn Bartle, DNP, CPNP-AC/PC, Duke Children’s Pediatric and Congenital Heart Center Mary Rummell, MN, RN, CNS, CPNP, FAHA, Oregon Health and Science University Introduction: Children born with hypoplastic left heart syndrome (HLHS) are at high risk for serious morbidity, growth failure and mortality during the time from discharge home after first stage HLHS palliation, until admission for the second stage surgical intervention. To improve outcomes and survival during this time, referred to as the “interstage period”, multiple strategies have been successfully implemented resulting in improved interstage survival. A critical element of interstage care is family education and training. Variations in practice and interstage home surveillance do exist but general guidelines are described below. Definitions: Possible newborn interventions for HLHS Figure 1: First stage surgical palliation procedures for hyoplastic left heart syndrome A. Norwood Procedure B. Norwood Procedure with C. Hybrid Stage I with BT shunt RV to PA shunt (“Sano”) Procedure Surgical: Norwood /Modified Blalock Taussig (BT) shunt procedure – the Norwood aortic reconstruction of the ascending aorta and arch consists of enlarging the ascending aorta by using part of the proximal native main pulmonary artery (PA) with other material (Gore-Tex or homograft material) to connect the PA to the aorta. Pulmonary blood flow is provided by a controlled shunt (BT shunt) usually made from Gore-Tex and connects the right subclavian artery to the right pulmonary artery. -

Long Term Consequences of the Fontan Procedure and How to Manage Them
ACCEPTED MANUSCRIPT Long Term Consequences of the Fontan Procedure and How to Manage Them Authors: W. Aaron Kay, MD1 Tabitha Moe, MD2 Blair Suter, MD3 Andrea Tennancour, NP1 Alice Chan, NP4 Richard A. Krasuski, MD5 Ali N. Zaidi, MD4 1. Indiana University School of Medicine, Krannert Institute of Cardiology, IN 2. University of Arizona School of Medicine, Phoenix, AZ 3. Indiana University School of Medicine, Departments of Medicine and Pediatrics, IN 4. Children’s Hospital at Montefiore, Montefiore Medical Center, Albert Einstein College of Medicine, NY 5. Duke University Health System, Durham, NC *Address reprint requests to: W. Aaron Kay, MD. 1801 N. Senate Blvd Suite 2000, Indianapolis IN. Email: [email protected] Emails of additional authors: Moe TG: [email protected] Suter BC: [email protected] Tennancour AE: [email protected] Chan A: [email protected] Krasuski RA: [email protected] Zaidi AN: [email protected] Disclosures: W. Aaron Kay: Nothing to disclose Tabitha Moe: Nothing to disclose Blair Suter, MD: Nothing to disclose Andrea Tennancour, NP: Nothing to disclose Alice Chan, NP: Nothing to disclose Ali N. Zaidi, MD: Nothing to disclose Richard A. Krasuski, MD: Dr. Krasuski serves a consultant and receives research funding from Actelion Pharmaceuticals.ACCEPTED He also serves as anMANUSCRIPT investigator for Edwards Lifesciences and is an unpaid member of the scientific advisory board for Ventripoint. ___________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Kay, W. A., Moe, T., Suter, B., Tennancour, A., Chan, A., Krasuski, R. A., & Zaidi, A. N. (2018). Long Term Consequences of the Fontan Procedure and How to Manage Them. -

Note to Users for Pedcsrs 2010 Data Collection
Note to Users for PedCSRS 2010 Data Collection The PedCSRS coding instructions and data element definitions issued for 2009 discharges will remain in effect for 2010 discharges. There are no changes to the data collection form, definitions or coding instructions. The attached document contains the current procedure codes that should be used for 2010 PedCSRS reporting. This includes the changes to procedure codes made for July 2009 Adult CSRS reporting. For discharge year 2010, use form DOH-2254p (1/09). Although the bottom of that form states “2009 Discharges,” the effective period for this form has been extended through December 2010 discharges. The file specification for 2010 PedCSRS data contains no changes from the 2009 file structure. The document has been updated only for dates and naming conventions. A copy of the current PedCSRS file specifications document can be obtained from the Cardiac Services Program. Reporting Schedule Pediatric CSRS data is reported quarterly by discharge date. It is due to the Cardiac Services Program two months after the end of the quarter. The 2010 reporting schedule is as follows. Quarter 1 (1/1/10 – 3/31/10 Discharges) due on or before May 31, 2010 Quarter 2 (4/1/10 – 6/30/10 Discharges) due on or before August 31, 2010 Quarter 3 (7/1/10 – 9/30/10 Discharges) due on or before November 30, 2010 Quarter 4 (10/1/10 – 12/31/10 Discharges) due on or before February 28, 2011 Limited extensions to the above deadlines will be granted on a case by case basis when warranted by extenuating circumstances. -

Mustard Procedure Br Heart J: First Published As 10.1136/Hrt.72.1.85 on 1 July 1994
Br Heart J 1994;72:85-88 85 Transcatheter stent implantation for recurrent pulmonary venous pathway obstruction after the Mustard procedure Br Heart J: first published as 10.1136/hrt.72.1.85 on 1 July 1994. Downloaded from Martin C K Hosking, Kenneth A Murdison, Walter J Duncan Abstract pathway obstruction reaching a maximum A 5 year old boy presented with obstruc- flow velocity of 2'0 m/s associated with a tion of the pulmonary venous pathway stenosis measuring 2-3 mm in diameter. four years after the Mustard procedure. Right ventricular dysfunction was a persistent A successful balloon dilatation ofthe pul- echocardiographic finding associated with an monary venous pathway was performed increase in left ventricle dimension in but the benefit was transient. Placement response to the rise of pulmonary arterial of a 10 mm balloon expandable intravas- pressure secondary to the pulmonary venous cular stent across the recurrent stenosis inflow obstruction. A significant systemic to resulted in complete relief ofthe obstruc- pulmonary venous chamber baffle leak mea- tion with prompt resolution ofthe clinical suring 5 mm x 4 mm with right to left shunting signs. The delivery system was modified was seen by Doppler colour flow mapping and to facilitate stent delivery. contributed to central cyanosis. Serial chest x ray films showed persistence of cardiomegaly (Br Heart 1994;72:85-88) with increasing evidence of pulmonary venous congestion. To avoid the risks of surgical repair in a Paediatric experience with transcatheter patient with impaired -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

Pericardiocentesis Versus Pericardiotomy for Malignant Pericardial Effusion: a Retrospective Comparison
MALIGNANT PERICARDIAL EFFUSION, Labbé et al. ORIGINAL ARTICLE Pericardiocentesis versus pericardiotomy for malignant pericardial effusion: a retrospective comparison C. Labbé MD,* L. Tremblay MD,* and Y. Lacasse MD MSc* ABSTRACT Background Treatment of malignant pericardial effusion remains controversial, because no randomized controlled trials have been conducted to determine the best approach, and results of retrospective studies have been inconsistent. The objective of the present study was to compare pericardiocentesis and pericardiotomy with respect to efficacy for preventing recurrence, and to determine, for those two procedures, diagnostic yields, complication rates, and effects on survival. We also aimed to identify clinical and procedural factors that could predict effusion recurrence. Methods We retrospectively assessed 61 patients who underwent a procedure for treatment of a malignant pericardial effusion at the Institut universitaire de cardiologie et de pneumologie de Québec between February 2004 and September 2013. Results Pericardiocentesis was performed in 42 patients, and pericardiotomy, in 19 patients. The effusion recurrence rate was significantly higher in patients treated with pericardiocentesis than with pericardiotomy (31.0% vs. 5.3%, p = 0.046). The diagnostic yield of the procedures was not significantly different (92.9% vs. 86.7%, p = 0.6). The overall rate of complications was similar in the two groups, as was the median overall survival (2.4 months vs. 2.6 months, p = 0.5). In univariate analyses, the procedure type was the only predictor of recurrence that approached statistical significance. Age, sex, type of cancer, presence of effusion at the time of cancer diagnosis, prior chest irradiation, tamponade upon presentation, and total volume of fluid removed did not influence the recurrence rate. -

Mustard Operation
Mustard Operation William G. Williams The natural history of infants with complete transposi- desaturation. Mustard reasoned that both vena cavae tion (TGA) is rapid deterioration and death within the could be deliberately diverted to the left atrium while first few months of life. The few babies who do survive allowing the pulmonary return to enter the right atrium, beyond the first year of life usually have an associated thereby correcting the circulation in patients with TGA. ventricular septal defect (VSD) and subsequently dete- The Mustard baffle operation was a simple, reproduc- riorate with progressive pulmonary vascular disease. ible, and elegant technique of transposing venous re- Intervention to improve the outlook for newborns turn. It was easier to learn than the Senning operation, with TGA began with surgical resection of the atrial and became the standard repair in most of the world. septum.' The enlarged atrial defect improves mixing of the pulmonary and systemic circulations, thereby in- creasing the systemic oxygen saturation. Balloon atrial Indications for the Mustard Operation in 1998 septostomy, as described by Rashkind and Miller in The atrial repair of TGA has been almost entirely 1966,2 replaced surgical septectomy. The balloon cath- replaced by the arterial switch operation. However, eter technique continues to provide effective emergency there are three situations were the Mustard operation palliation for the newborn with TGA. may be indicated: In the early 1950s, a number of surgeons, including 1. For infants with isolated TGA who first present M~stard,~attempted arterial repair of TGA in infants, after the neonatal period, the Mustard operation is an all without success until Jatene's report in 1978.4 In alternative to the two-stage arterial switch operation, 1954, Albert5 proposed a surgical technique to trans- ie, a preliminary pulmonary artery banding to prepare pose the venous inflow within the atria to correct TGA. -

The Arterial Switch Operation for Transposition of the Great Arteries
The Arterial Switch Operation for Transposition of the Great Arteries Richard A. Jonas Transposition of the great arteries, together with tetral- Ijased on the fact that (luring fetal life hoth ventricles ogy of Fallot, is one of the most common congenital are exposed to the same pressure load. Thus, the left cardiac anomalies resulting in cyanosis. It is hardly ventricle is prepared at birth. However, hecause pulnio- surprising, therefore, that shortly after the introduc- nary resistance falls ra1)idlj within 6 to 8 weeks ancl tion of cardiopulmonary hypass in the early 195Os, perhaps as quicltly as within 2 to 4 weeks, the left attempts were made at anatomical correction of transpo- ventricle is no longer prepared. Iiitroduction of an sition, ie, the arterial switch procedure. These early essentially elective complex neonatal operation in 1983 attempts were uniformly unsuccessful for a number of initially met with consitlerahle resistance, particularly reasons : 1)ecause hy this time the surgical mortality for the atrial 1. Microvascular techniques were not adequately level procedures had reached very low levels. However, developed for delicate coronary artery transfer. a prospectivr study conducted Ijy the Congenital Heart 2. Pulmonary vascular disease is common in transpo- Surgeons Society‘ showed that the overall survival of an sition beyond the first year of life. entire population of patients with transposition en- 3. Surgeons failed to appreciate that in the presence rolled in the neonatal period was superior with the of an intact ventricular septum, the left ventricle was neonatal arterial switch approach because of the previ- inadequately prepared to acntely take over the pressure ously uncaptured deaths that occurred before atrial load of the systemic circulation.