Risk Factors for Mortality After the Norwood Procedureq
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
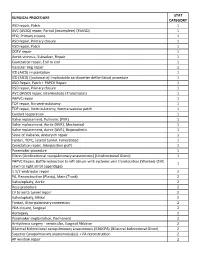
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Stra
Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Strawn, BSN, RN, CPN, Children’s Hospital & Medical Center, Omaha, Nebraska Jo Ann Nieves MSN, CPN, ARNP, PNP-BC, FAHA, Miami Children’s Hospital Bronwyn Bartle, DNP, CPNP-AC/PC, Duke Children’s Pediatric and Congenital Heart Center Mary Rummell, MN, RN, CNS, CPNP, FAHA, Oregon Health and Science University Introduction: Children born with hypoplastic left heart syndrome (HLHS) are at high risk for serious morbidity, growth failure and mortality during the time from discharge home after first stage HLHS palliation, until admission for the second stage surgical intervention. To improve outcomes and survival during this time, referred to as the “interstage period”, multiple strategies have been successfully implemented resulting in improved interstage survival. A critical element of interstage care is family education and training. Variations in practice and interstage home surveillance do exist but general guidelines are described below. Definitions: Possible newborn interventions for HLHS Figure 1: First stage surgical palliation procedures for hyoplastic left heart syndrome A. Norwood Procedure B. Norwood Procedure with C. Hybrid Stage I with BT shunt RV to PA shunt (“Sano”) Procedure Surgical: Norwood /Modified Blalock Taussig (BT) shunt procedure – the Norwood aortic reconstruction of the ascending aorta and arch consists of enlarging the ascending aorta by using part of the proximal native main pulmonary artery (PA) with other material (Gore-Tex or homograft material) to connect the PA to the aorta. Pulmonary blood flow is provided by a controlled shunt (BT shunt) usually made from Gore-Tex and connects the right subclavian artery to the right pulmonary artery. -

Long Term Consequences of the Fontan Procedure and How to Manage Them
ACCEPTED MANUSCRIPT Long Term Consequences of the Fontan Procedure and How to Manage Them Authors: W. Aaron Kay, MD1 Tabitha Moe, MD2 Blair Suter, MD3 Andrea Tennancour, NP1 Alice Chan, NP4 Richard A. Krasuski, MD5 Ali N. Zaidi, MD4 1. Indiana University School of Medicine, Krannert Institute of Cardiology, IN 2. University of Arizona School of Medicine, Phoenix, AZ 3. Indiana University School of Medicine, Departments of Medicine and Pediatrics, IN 4. Children’s Hospital at Montefiore, Montefiore Medical Center, Albert Einstein College of Medicine, NY 5. Duke University Health System, Durham, NC *Address reprint requests to: W. Aaron Kay, MD. 1801 N. Senate Blvd Suite 2000, Indianapolis IN. Email: [email protected] Emails of additional authors: Moe TG: [email protected] Suter BC: [email protected] Tennancour AE: [email protected] Chan A: [email protected] Krasuski RA: [email protected] Zaidi AN: [email protected] Disclosures: W. Aaron Kay: Nothing to disclose Tabitha Moe: Nothing to disclose Blair Suter, MD: Nothing to disclose Andrea Tennancour, NP: Nothing to disclose Alice Chan, NP: Nothing to disclose Ali N. Zaidi, MD: Nothing to disclose Richard A. Krasuski, MD: Dr. Krasuski serves a consultant and receives research funding from Actelion Pharmaceuticals.ACCEPTED He also serves as anMANUSCRIPT investigator for Edwards Lifesciences and is an unpaid member of the scientific advisory board for Ventripoint. ___________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Kay, W. A., Moe, T., Suter, B., Tennancour, A., Chan, A., Krasuski, R. A., & Zaidi, A. N. (2018). Long Term Consequences of the Fontan Procedure and How to Manage Them. -

Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome
SURGERY | REVIEW Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome Hideyuki Kato, MD, Osami Honjo, MD, PhD, Glen Van Arsdell, MD, Christopher A. Caldarone, MD Division of Cardiovascular Surgery, Hospital for Sick Children, Labatt Family Heart Center Received 12/12/2011, Reviewed 27/12/2011, Accepted 1/1/2012 Key words: Hybrid strategy, Norwood strategy, single ventricle, hypoplastic left heart syndrome DOI: 10.5083/ejcm.20424884.70 ABSTRACT CORRESPONDENCE Current surgical palliation for neonates with single ventricle physiology includes Norwood-based Christopher A. Caldarone, MD, and Hybrid-based surgical strategies. When transplantation is not available, clinicians must choose Professor and Chair, Division of Cardiac Surgery, between these two strategies with distinctly different learning curves and risk profiles. The Norwood University of Toronto, strategy has evolved over several decades while the rising popularity of the Hybrid strategy is a Watson Family Chair in much more recent addition to our therapeutic options. Based upon the premise that avoiding cardio Cardiovascular Science – pulmonary bypass in the neonatal period and deferral of aortic arch reconstruction until a second Labatt Family Heart Center, stage procedure have an important influence on outcomes, the Hybrid strategy has compelling Hospital for Sick Children, theoretical advantages and disadvantages in comparison to the Norwood strategy. The purpose 555 University Avenue, of this review is to summarise the currently available data to support -

The Intensive Care of Infants with Hypoplastic Left Heart Syndrome
F97 Arch Dis Child Fetal Neonatal Ed: first published as 10.1136/adc.2004.064337 on 21 February 2005. Downloaded from RECENT ADVANCES The intensive care of infants with hypoplastic left heart syndrome U Theilen, L Shekerdemian ............................................................................................................................... Arch Dis Child Fetal Neonatal Ed 2005;90:F97–F102. doi: 10.1136/adc.2004.051276 Until a little over two decades ago, hypoplastic left heart excessive pulmonary blood flow, an important subgroup may have inadequate inter-atrial mix- syndrome was considered an inoperable and fatal ing due to a restrictive atrial septal defect (ASD). condition, with most deaths occurring in early infancy, and This results in global hypoperfusion, with pro- almost all of those affected dying before their first birthday. found hypoxaemia and acidosis, severe pulmon- ary venous hypertension, and overwhelming However, the advent of surgical palliation and advances in pulmonary venous congestion on chest x ray. peri-operative care, have offered hope to these patients These infants are generally unresponsive to and their families. measures aimed at improving pulmonary flow such as aggressive mechanical ventilation with ........................................................................... high inspired oxygen fractions or nitric oxide, and if left untreated they rapidly develop ypoplastic left heart syndrome (HLHS) is a progressive pulmonary venous hypertension. continuum which can affect all left sided This is associated with increased mortality in Hcardiac structures, from the mitral valve to infants with HLHS.34 the aortic arch. Since Norwood’s first description A restrictive ASD requires urgent intervention. of surgical palliation in 1981,1 HLHS has been If this has been diagnosed antenatally, delivery managed either by staged palliation in the should be planned in a centre able to urgently majority of cases, or, in a minority, by primary institute cardiopulmonary bypass. -

Norwood/Batista Operation for a Newborn with Dilated Myopathy of the Left Ventricle
612 Brief communications The Journal of Thoracic and Cardiovascular Surgery September 2000 NORWOOD/BATISTA OPERATION FOR A NEWBORN WITH DILATED MYOPATHY OF THE LEFT VENTRICLE Richard D. Mainwaring, MD, Regina M. Healy, BS, John D. Murphy, MD, and William I. Norwood, MD, PhD, Wilmington, Del Partial left ventriculectomy for dilated cardiomyopathy was contractile function. The variable results that have been first reported by Batista and associates1 in 1996. The rationale reported in the adult literature may reflect patient selection for this procedure is the increase in left ventricular cavity size according to the reversibility or recoverability of the underly- in the absence of compensatory left ventricular wall thickness ing disease process. that is observed in dilated cardiomyopathy. This combination Despite the substantial worldwide experience with the of factors results in an increase in wall stress per unit muscle Batista procedure in adult patients, there is limited experience mass, as predicted by the LaPlace equation. As wall stress with this procedure in children. The current case report increases, mechanical load eventually becomes nonsustain- describes the treatment of a patient in whom the diagnosis of able and contributes to further dilation of the ventricle. Partial dilated cardiomyopathy was made in utero. left ventriculectomy has been advocated as a method of Clinical summary. A female infant was recognized in restoring the balance between cavity size and wall thickness. utero as having a dilated, poorly functioning left ventricle. Fundamental to this concept is the assumption that the left Labor was induced at 36 weeks’ gestation, and she was deliv- ventricular muscle is intrinsically normal or has recoverable ered by normal, spontaneous, vaginal delivery. -

Index2 Rev.Pdf
INDEX A American Association of Thoracic Surgery, 19 Abiomed total artificial heart, 197 American College of Cardiology, 69 ACE inhibitors, 118 Amosov, Nikolay, 30–31 adenovirus, 199 anasarca, 63 Agnew Clinic, The, 16 anastomosis, 222 Akutsu, Dr. Tetsuzo, 186, 189 aneurysms alcohol aortic, 223–225 blood lipids, relationship between, catheter treatment, 225 53–54 challenges, surgical, 224 breast cancer link, 55 diagnosis, 224 consumption, 51, 53–54 discovery, 223 dangers, 55 dissection, 226–227 French Paradox, 52 stent treatment, 225 life expectancy, 56 stent-graft treatment, 225–226, mortality rates, 55 231–232 pattern of consumption, 54 surgical treatment, 230 red wine consumption, 54 symptoms, 223 scientific data summary, 55 types, 223–225 wine drinker profile vs. beer drinker angina pectoris, 42, 159 profile, 54 classic symptoms, 60 alpha-linoleic acid, 49 signaling impending heart attack, 61 alveoli, 35 angiogenesis, 199 ambulatory electrocardiogram monitoring, angiography, coronary, 86 74 angiography, pulmonary, 86 284 INDEX antibiotic protection, dental surgery, 57 arterial blood samples, 81 anticoagulant use, 134 arterial oxygen levels, 81 aorta, 32, 38, 96 arterial revascularization, 241 aortic arch, 103 arterial system, 221 aortic incompetence, 159 artery, definition, 33 aortic valve, 35, 64 artherosclerosis, 45 aortic valve disease, 158–159 artificial heart, 186 aortic valve incompetence, 159–160 Akutsu, Dr. Tetsuzo, 186 aortic valve stenosis, 64, 158–159 Clark, Barney, 188 aortomyoplasty Cooley, Dr. Denton, 186 advantages, 193 DeBakey, Dr. Michael, 186 application, 193 development, 186 definition, 192 DeVries, Dr. William, 188 Arbulu, Dr. Agustin, 163 first implantation, 186 argyria, 66 flaws, 200 arrhythmia, 64, 74 Jarvik, Dr. Robert, 188 advancements in studies, 217 Jarvik-7, 187–188 atrial, 140 Jarvik-2000, 201 atrial fibrillation, 215, 218 Kolff, Dr. -

Pediatric Cardiac Imaging Guidelines V1.0
CLINICAL GUIDELINES Pediatric Cardiac Imaging Policy Version 1.0 Effective February 14, 2020 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Pediatric Cardiac Imaging Guidelines V1.0 Pediatric Cardiac Imaging Guidelines PEDCD-1: General Guidelines 3 PEDCD-2: Congenital Heart Disease 9 PEDCD-3: Heart Murmur 14 PEDCD-4: Chest Pain 16 PEDCD-5: Syncope 19 PEDCD-6: Kawasaki Disease 22 PEDCD-7: Pediatric Pulmonary Hypertension 28 PEDCD-8: Echocardiography – Other Indications 31 PEDCD-9: Cardiac MRI – Other Indications 36 PEDCD-10: CT Heart and Coronary Computed Tomography Angiography (CCTA) – Other Indications 41 PEDCD-11: Cardiac Catheterization 46 Procedure -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

Table of Contents Introduction
Congenital Heart Surgery Database v.3.3 effective July 1, 2015 Training Manual October 2017 Table of Contents Introduction ................................................................................................................................................................2 1. Administrative ...............................................................................................................................................2 2. Demographics ...............................................................................................................................................5 3. Noncardiac Congenital Anatomic Abnormalities ....................................................................................... 21 4. Chromosomal Abnormalities ..................................................................................................................... 22 5. Syndromes ................................................................................................................................................. 23 6. Hospitalization ........................................................................................................................................... 24 7. Preoperative Factors .................................................................................................................................. 28 8. Diagnosis .................................................................................................................................................... 30 9. Procedures ................................................................................................................................................ -

CVS and ICU Management
Disclaimer: The Great Ormond Street Paediatric Intensive Care Training Programme was developed in 2004 by the clinicians of that Institution, primarily for use within Great Ormond Street Hospital and the Children’s Acute Transport Service (CATS). The written information (known as Modules) only forms a part of the training programme. The modules are provided for teaching purposes only and are not designed to be any form of standard reference or textbook. The views expressed in the modules do not necessarily represent the views of all the clinicians at Great Ormond Street Hospital and CATS. The authors have made considerable efforts to ensure the information contained in the modules is accurate and up to date. The modules are updated annually. Users of these modules are strongly recommended to confirm that the information contained within them, especially drug doses, is correct by way of independent sources. The authors accept no responsibility for any inaccuracies, information perceived as misleading, or the success of any treatment regimen detailed in the modules. The text, pictures, images, graphics and other items provided in the modules are copyrighted by “Great Ormond Street Hospital” or as appropriate, by the other owners of the items. Copyright 2004-2005 Great Ormond Street Hospital. All rights reserved. CVS: ICU Management of HLHS and Single Ventricle Physiology Allan Goldman, April 2006 Updated Adrian Plunkett, February 2007, Timothy Thiruchelvam, May 2009 Associated clinical guidelines/protocols: • Cardiac ICU Manual Fundamental