GLYCOLYSIS • All Cells Carry out Glycolysis • the Entire Process Occurs in Cytoplasm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Indications for a Central Role of Hexokinase Activity in Natural Variation of Heat Acclimation in Arabidopsis Thaliana
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 14 June 2020 doi:10.20944/preprints202006.0169.v1 Article Indications for a central role of hexokinase activity in natural variation of heat acclimation in Arabidopsis thaliana Vasil Atanasov §, Lisa Fürtauer § and Thomas Nägele * LMU Munich, Plant Evolutionary Cell Biology, Großhaderner Str. 2-4, 82152 Planegg, Germany § Authors contributed equally * Correspondence: [email protected] Abstract: Diurnal and seasonal changes of abiotic environmental factors shape plant performance and distribution. Changes of growth temperature and light intensity may vary significantly on a diurnal, but also on a weekly or seasonal scale. Hence, acclimation to a changing temperature and light regime is essential for plant survival and propagation. In the present study, we analyzed photosynthetic CO2 assimilation and metabolic regulation of the central carbohydrate metabolism in two natural accessions of Arabidopsis thaliana originating from Russia and south Italy during exposure to heat and a combination of heat and high light. Our findings indicate that it is hardly possible to predict photosynthetic capacities to fix CO2 under combined stress from single stress experiments. Further, capacities of hexose phosphorylation were found to be significantly lower in the Italian than in the Russian accession which could explain an inverted sucrose-to-hexose ratio. Together with the finding of significantly stronger accumulation of anthocyanins under heat/high light these observations indicate a central role of hexokinase activity in stabilization of photosynthetic capacities within a changing environment. Keywords: photosynthesis; carbohydrate metabolism; hexokinase; heat acclimation; environmental changes; natural variation; high light; combined stress. 1. Introduction Changes of growth temperature and light intensity broadly affect plant molecular, physiological and developmental processes. -

• Glycolysis • Gluconeogenesis • Glycogen Synthesis
Carbohydrate Metabolism! Wichit Suthammarak – Department of Biochemistry, Faculty of Medicine Siriraj Hospital – Aug 1st and 4th, 2014! • Glycolysis • Gluconeogenesis • Glycogen synthesis • Glycogenolysis • Pentose phosphate pathway • Metabolism of other hexoses Carbohydrate Digestion! Digestive enzymes! Polysaccharides/complex carbohydrates Salivary glands Amylase Pancreas Oligosaccharides/dextrins Dextrinase Membrane-bound Microvilli Brush border Maltose Sucrose Lactose Maltase Sucrase Lactase ‘Disaccharidase’ 2 glucose 1 glucose 1 glucose 1 fructose 1 galactose Lactose Intolerance! Cause & Pathophysiology! Normal lactose digestion Lactose intolerance Lactose Lactose Lactose Glucose Small Intestine Lactase lactase X Galactose Bacteria 1 glucose Large Fermentation 1 galactose Intestine gases, organic acid, Normal stools osmotically Lactase deficiency! active molecules • Primary lactase deficiency: อาการ! genetic defect, การสราง lactase ลด ลงเมออายมากขน, พบมากทสด! ปวดทอง, ถายเหลว, คลนไสอาเจยนภาย • Secondary lactase deficiency: หลงจากรบประทานอาหารทม lactose acquired/transient เชน small bowel เปนปรมาณมาก เชนนม! injury, gastroenteritis, inflammatory bowel disease! Absorption of Hexoses! Site: duodenum! Intestinal lumen Enterocytes Membrane Transporter! Blood SGLT1: sodium-glucose transporter Na+" Na+" •! Presents at the apical membrane ! of enterocytes! SGLT1 Glucose" Glucose" •! Co-transports Na+ and glucose/! Galactose" Galactose" galactose! GLUT2 Fructose" Fructose" GLUT5 GLUT5 •! Transports fructose from the ! intestinal lumen into enterocytes! -

Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1
[CANCER RESEARCH 34, 1439-1446, June 1974] Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1 Francis A. Farina,2 Jennie B. Shatton, Harold P. Morris, and Sidney Weinhouse The Fels Research Institute and the Department of Biochemistry, Temple University School oj Medicine, Philadelphia. Pennsylvania IV140 (F. A. F., J. B. S.. S. W.\, and the Department of Biochemistry. Howard University School of Medicine. Washington. D. C. 20001 [H. P. M .\ SUMMARY 23, 32, 41, 57). These alterations involve the replacement of those isozymes that are under dietary and hormonal control Pyruvate kinase (PK) (EC 2.7.1.40) isozymes were assayed by the host, and that have important metabolic functions in in normal rat liver and a series of transplantable rat the adult differentiated liver by other isozymes which are hepatomas ranging widely in growth rate and degree of normally either low in, or absent from, the adult tissue. As differentiation, with the use of gradient elution by chloride part of an ongoing study of this phenomenon, we have ion from columns of DEAE-cellulose. In agreement with examined the alteration of PK3 (EC 2.7.1.40) isozymes in other studies, three noninterconvertible forms were found in the Morris hepatomas (30. 31), a series of chemically rat tissues: isozyme I, the major form in adult rat liver: induced, transplantable rat hepatomas ranging widely in isozyme II, the sole form in heart and skeletal muscle: and growth rate and degree of differentiation. This enzyme isozyme III, the sole form in poorly differentiated hepato occupies a key position in the metabolism of cells and, as we mas, the major form in normal kidney and lung, and the pointed out previously (27, 28. -

Labeled in Thecourse of Glycolysis, Since Phosphoglycerate Kinase
THE STATE OF MAGNESIUM IN CELLS AS ESTIMATED FROM THE ADENYLATE KINASE EQUILIBRIUM* BY TRWIN A. RoSE THE INSTITUTE FOR CANCER RESEARCH, PHILADELPHIA Communicated by Thomas F. Anderson, August 30, 1968 Magnesium functions in many enzymatic reactions as a cofactor and in com- plex with nucleotides acting as substrates. Numerous examples of a possible regulatory role of Mg can be cited from studies with isolated enzymes,'- and it is known that Mg affects the structural integrity of macromolecules such as trans- fer RNA" and functional elements such as ribosomes.'0 The major problem in translating this information on isolated preparations to the functioning cell is the difficulty in determining the distribution of Mg and the nucleotides among the free and complexed forms that function in the region of the cell for which this information is desired. Nanningall based an attempt to calculate the free Mg2+ and Ca2+ ion concentrations of frog muscle on the total content of these metals and of the principal known ligands (adenosine 5'-triphosphate (ATP), creatine-P, and myosin) and the dissociation constants of the complexes. However, this method suffers from the necessity of evaluating the contribution of all ligands as well as from the assumption that all the known ligands are contributing their full complexing capacity. During studies concerned with the control of glycolysis in red cells and the control of the phosphoglycerate kinase step in particular, it became important to determine the fractions of the cell's ATP and adenosine 5'-diphosphate (ADP) that were present as Mg complexes. Just as the problem of determining the distribution of protonated and dissociated forms of an acid can be solved from a knowledge of pH and pKa of the acid, so it would be possible to determine the liganded and free forms of all rapidly established Mg complexes from a knowledge of Mg2+ ion concentration and the appropriate dissociation constants. -

Pyruvate Kinase: Function, Regulation and Role in Cancer
Pyruvate kinase: Function, regulation and role in cancer The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Israelsen, William J., and Matthew G. Vander Heiden. “Pyruvate Kinase: Function, Regulation and Role in Cancer.” Seminars in Cell & Developmental Biology 43 (2015): 43–51. As Published http://dx.doi.org/10.1016/j.semcdb.2015.08.004 Publisher Elsevier Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/105833 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ HHS Public Access Author manuscript Author Manuscript Author ManuscriptSemin Cell Author Manuscript Dev Biol. Author Author Manuscript manuscript; available in PMC 2016 August 13. Published in final edited form as: Semin Cell Dev Biol. 2015 July ; 43: 43–51. doi:10.1016/j.semcdb.2015.08.004. Pyruvate kinase: function, regulation and role in cancer William J. Israelsena,1,* and Matthew G. Vander Heidena,b,* aKoch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA bDepartment of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA 02115, USA Abstract Pyruvate kinase is an enzyme that catalyzes the conversion of phosphoenolpyruvate and ADP to pyruvate and ATP in glycolysis and plays a role in regulating cell metabolism. There are four mammalian pyruvate kinase isoforms with unique tissue expression patterns and regulatory properties. The M2 isoform of pyruvate kinase (PKM2) supports anabolic metabolism and is expressed both in cancer and normal tissue. The enzymatic activity of PKM2 is allosterically regulated by both intracellular signaling pathways and metabolites; PKM2 thus integrates signaling and metabolic inputs to modulate glucose metabolism according to the needs of the cell. -
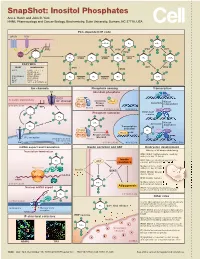
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Interaction of 6-Phosphofructo-2-Kinase/Fructose-2,6- Bisphosphatase (PFK-2/Fbpase-2) with Glucokinase Activates Glucose Phospho
Interaction of 6-Phosphofructo-2-Kinase/Fructose-2,6- Bisphosphatase (PFK-2/FBPase-2) With Glucokinase Activates Glucose Phosphorylation and Glucose Metabolism in Insulin-Producing Cells Laura Massa,1 Simone Baltrusch,1 David A. Okar,2,3 Alex J. Lange,2 Sigurd Lenzen,1 and Markus Tiedge1 The bifunctional enzyme 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase (PFK-2/FBPase-2) was re- cently identified as a new intracellular binding partner he enzyme glucokinase (GK) plays a pivotal role for glucokinase (GK). Therefore, we studied the impor- in the recognition of glucose in pancreatic tance of this interaction for the activity status of GK -cells and the regulation of glucose metabolism and glucose metabolism in insulin-producing cells by Tin the liver (1–7). In pancreatic -cells, GK acts overexpression of the rat liver and pancreatic islet as a glucose sensor and catalyzes the rate-limiting step for isoforms of PFK-2/FBPase-2. PFK-2/FBPase-2 overex- initiation of glucose-induced insulin secretion (6). GK is pression in RINm5F-GK cells significantly increased the regulated in a complex manner in pancreatic -cells by GK activity by 78% in cells expressing the islet isoform, posttranslational modifications of the enzyme protein that by 130% in cells expressing the liver isoform, and by mainly depend on the intracellular glucose concentration 116% in cells expressing a cAMP-insensitive liver S32A/ (8–13). These posttranslational mechanisms of GK activity H258A double mutant isoform. Only in cells overex- regulation are comprised of conformational changes pressing the wild-type liver PFK-2/FBPase-2 isoform (14,15), sulfhydryl-group conversions (16–18), and inter- was the increase of GK activity abolished by forskolin,  apparently due to the regulatory site for phosphoryla- actions with -cell matrix proteins (13,19), insulin gran- tion by a cAMP-dependent protein kinase. -

New Nucleotide Sequence Data on the EMBL File Server
.=) 1991 Oxford University Press Nucleic Acids Research, Vol. 19, No. 2 413 New nucleotide sequence data on the EMBL File Server October 30, 1990 to November 13, 1990 New nucleotide sequence data, available from the EMBL File Server, (see Stoehr, P.J. and Omond, R.A. (1989) Nucleic Acids Res., 17 (16), 6763 -6764), are reported below. Availability of all the newest sequence data is the result of collaboration between.the EMBL Data Library and GenBank' , and data are supplied regularly by both groups. Updates to existing data are not reported here. This report has been prepared by the EMBL Data Library. PRIMATES: Human casein kinase II alpha subunit mRNA, complete cds. Human specific HS5 DNA Lozeman F.J., Litchfield D.W., Piening C., Takio K., Walsh Ueda S., Washio K., Kurosaki K.; K.A., Krebs E.G.; Genomics 8:7-12(1990). X17579 Biochemistry 29:8436-8447(1990). M55268 Human mRNA for heat shock protein HSP27 Human mRNA for actin-binding protein (ABP-280) Carper S.W., Rocheleau T.A., Storm F.K.; Gorlin J.B., Yamin R., Egan S., Stewart M., Stossel T.P., Nucleic Acids Res. 18:6457-6457(1990). X54079 Kwiatkowski D.J., Hartwig J.H.; J. Cell Biol. 111:1089-1105(1990). X53416 Human Ig germline H-chain D-region Dxpl and Dxp'1 genes, 3' Human amiloride-binding protein, complete cds. end. Barbry P., Champe M., Chassande O., Munemitsu S., Champigny Huang C., Stollar B.David.; G., Lingueglia E., Maes P., Frelin C., Tartar A., Ullrich A., Unpublished. M37485 Lazdunski M.; Proc. Natl. Acad. -

Cdna Sequence and Tissue Expression Analysis of Glucokinase from Liver of Grass Carp (Ctenopharyngodon Idella )
African Journal of Biotechnology Vol. 11(3), pp. 534-542, 10 January, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.3174 ISSN 1684–5315 © 2012 Academic Journals Full Length Research Paper cDNA sequence and tissue expression analysis of glucokinase from liver of grass carp (Ctenopharyngodon idella ) CHENG Han-liang*, JI Nan-jing, PENG Yong-xing, SHEN Xin, XU Jian-he, DONG Zhi-guo and WU Chen-chen Jiangsu Key Laboratory of Marine Biotechnology, Huaihai Institute of Technology, Lianyungang 222005, People’s Republic of China. Accepted 19 December, 2011 A full-length cDNA coding glucokinase (GK) was cloned from the liver of grass carp ( Ctenopharyngodon idella ) by reverse transcriptase-polymerase chain reaction (RT-PCR) and rapid amplification of cDNA ends methods. The cDNA obtained was 2066 bp exclusive of poly (A) residues with a 1431 bp open reading frame encoding 476 amino acids. The GK protein has a calculated molecular weight of 53.7 kDa and isoelectric point of 5.11. Some conserved functional sites were found including one conserved hexokinase signature sequence Leu 156 -Phe 181 ; two N-linked glycosylation sites Asn 176 and Asn 214 ; one cell attachment sequence Arg 202 -Asp 204 ; one glycosaminoglycan attachment site Ser455 -Gly 458 . The amino acid sequence has a high similarity to GK of other species, the percent identity compared with topmouth culter, common carp, human and rat are 98.1, 96.8, 80.3 and 79.8%, respectively. Tissue distribution of GK mRNA in brain, mesenteric adipose tissue, spleen, white muscle and liver of grass carp was analyzed by SYBR real-time fluorescence quantitative RT-PCR method using β-actin as an internal control for cDNA normalization. -

Prevalence of Pyruvate Kinase Deficiency
P3513 Prevalence of Red Cell Pyruvate Kinase Deficiency: A Systematic Literature Review Mike Storm1, Matthew H Secrest2*, Courtney Carrington2, Deb Casso2, Keely Gilroy1, Leanne Pladson1, Audra N Boscoe1 1Agios Pharmaceuticals Inc., Cambridge, MA, USA; 2IQVIA Epidemiology & Drug Safety, Seattle, WA and Cambridge, MA, USA; *Affiliation at the time research was conducted BACKGROUND METHODS (continued) RESULTS (continued) RESULTS (continued) • Pyruvate Kinase (PK) deficiency is a rare congenital hemolytic anemia Exclusion Criteria The remaining 34 studies were grouped based on methods and study Among these 4 studies, an important distinction was made between studies characterized by diminished activity of the PK enzyme in red blood population (Table 1). reporting diagnosed prevalence (n=3) and overall disease prevalence cells (RBC).1 • Non-human studies; (diagnosed and undiagnosed PK deficiency; n=1). Table 1. Distribution of extracted studies by type of study (n=34) • Low PK enzyme activity can lead to lifelong chronic hemolysis with • Publications that were not the primary report of the data • Two studies estimated diagnosed PK deficiency prevalence as 3.2 per associated symptoms and complications such as anemia, jaundice, (e.g., literature reviews); Type of study Number million4 and 8.5 per million5 by identifying diagnosed PK deficiency cases of studies gallstones, splenectomy and associated thrombosis, iron overload, and • Studies of PK deficiency prevalence/incidence conducted within a source from source populations of known size. liver cirrhosis.2 Population-based prevalence 2 population of patients with symptoms of PK deficiency such as anemia • We estimated the prevalence of diagnosed PK deficiency in a general • PK deficiency is caused by compound heterozygosity or homozygosity for or jaundice; Molecular PKLR screening in a general population 5 population to be 6.5 per million6 using data from another high-quality study 3 one or more of the >300 known mutations to the PKLR gene. -

Table S1. List of Oligonucleotide Primers Used
Table S1. List of oligonucleotide primers used. Cla4 LF-5' GTAGGATCCGCTCTGTCAAGCCTCCGACC M629Arev CCTCCCTCCATGTACTCcgcGATGACCCAgAGCTCGTTG M629Afwd CAACGAGCTcTGGGTCATCgcgGAGTACATGGAGGGAGG LF-3' GTAGGCCATCTAGGCCGCAATCTCGTCAAGTAAAGTCG RF-5' GTAGGCCTGAGTGGCCCGAGATTGCAACGTGTAACC RF-3' GTAGGATCCCGTACGCTGCGATCGCTTGC Ukc1 LF-5' GCAATATTATGTCTACTTTGAGCG M398Arev CCGCCGGGCAAgAAtTCcgcGAGAAGGTACAGATACGc M398Afwd gCGTATCTGTACCTTCTCgcgGAaTTcTTGCCCGGCGG LF-3' GAGGCCATCTAGGCCATTTACGATGGCAGACAAAGG RF-5' GTGGCCTGAGTGGCCATTGGTTTGGGCGAATGGC RF-3' GCAATATTCGTACGTCAACAGCGCG Nrc2 LF-5' GCAATATTTCGAAAAGGGTCGTTCC M454Grev GCCACCCATGCAGTAcTCgccGCAGAGGTAGAGGTAATC M454Gfwd GATTACCTCTACCTCTGCggcGAgTACTGCATGGGTGGC LF-3' GAGGCCATCTAGGCCGACGAGTGAAGCTTTCGAGCG RF-5' GAGGCCTGAGTGGCCTAAGCATCTTGGCTTCTGC RF-3' GCAATATTCGGTCAACGCTTTTCAGATACC Ipl1 LF-5' GTCAATATTCTACTTTGTGAAGACGCTGC M629Arev GCTCCCCACGACCAGCgAATTCGATagcGAGGAAGACTCGGCCCTCATC M629Afwd GATGAGGGCCGAGTCTTCCTCgctATCGAATTcGCTGGTCGTGGGGAGC LF-3' TGAGGCCATCTAGGCCGGTGCCTTAGATTCCGTATAGC RF-5' CATGGCCTGAGTGGCCGATTCTTCTTCTGTCATCGAC RF-3' GACAATATTGCTGACCTTGTCTACTTGG Ire1 LF-5' GCAATATTAAAGCACAACTCAACGC D1014Arev CCGTAGCCAAGCACCTCGgCCGAtATcGTGAGCGAAG D1014Afwd CTTCGCTCACgATaTCGGcCGAGGTGCTTGGCTACGG LF-3' GAGGCCATCTAGGCCAACTGGGCAAAGGAGATGGA RF-5' GAGGCCTGAGTGGCCGTGCGCCTGTGTATCTCTTTG RF-3' GCAATATTGGCCATCTGAGGGCTGAC Kin28 LF-5' GACAATATTCATCTTTCACCCTTCCAAAG L94Arev TGATGAGTGCTTCTAGATTGGTGTCggcGAAcTCgAGCACCAGGTTG L94Afwd CAACCTGGTGCTcGAgTTCgccGACACCAATCTAGAAGCACTCATCA LF-3' TGAGGCCATCTAGGCCCACAGAGATCCGCTTTAATGC RF-5' CATGGCCTGAGTGGCCAGGGCTAGTACGACCTCG -

Yeast As a Tool to Understand the Significance of Human Disease
G C A T T A C G G C A T genes Review Yeast as a Tool to Understand the Significance of Human Disease-Associated Gene Variants Tiziana Cervelli and Alvaro Galli * Yeast Genetics and Genomics Group, Laboratory of Functional Genetics and Genomics, Institute of Clinical Physiology CNR, Via Moruzzi 1, 56125 Pisa, Italy; [email protected] * Correspondence: [email protected] Abstract: At present, the great challenge in human genetics is to provide significance to the growing amount of human disease-associated gene variants identified by next generation DNA sequencing technologies. Increasing evidences suggest that model organisms are of pivotal importance to addressing this issue. Due to its genetic tractability, the yeast Saccharomyces cerevisiae represents a valuable model organism for understanding human genetic variability. In the present review, we show how S. cerevisiae has been used to study variants of genes involved in different diseases and in different pathways, highlighting the versatility of this model organism. Keywords: yeast; Saccharomyces cerevisiae; functional assays; Mendelian disease; cancer; human gene variants 1. Introduction The advent of next generation DNA sequences technologies allows us to identify Citation: Cervelli, T.; Galli, A. Yeast individual genetic variants, and this revolutionized the strategy for discovering the cause as a Tool to Understand the of several genetic and multifactorial diseases. The association between genetic variants Significance of Human and disease risk can potentially overturn our understanding of common disease. The Disease-Associated Gene Variants. discovery of pathways and processes that are causally involved in a disease, so far not Genes 2021, 12, 1303. https:// linked, may open the way towards the development of targeted therapies and prevention doi.org/10.3390/genes12091303 strategies.