Ceramides: Nutrient Signals That Drive Hepatosteatosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P53 and Ceramide As Collaborators in the Stress Response
Int. J. Mol. Sci. 2013, 14, 4982-5012; doi:10.3390/ijms14034982 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review p53 and Ceramide as Collaborators in the Stress Response Rouba Hage-Sleiman 1,2,*, Maria O. Esmerian 1,2, Hadile Kobeissy 2 and Ghassan Dbaibo 1,2 1 Department of Pediatrics and Adolescent Medicine, Division of Pediatric Infectious Diseases, Faculty of Medicine, American University of Beirut, P.O. Box 11-0236 Riad El Solh, 1107 2020 Beirut, Lebanon; E-Mails: [email protected] (M.O.E.); [email protected] (G.D.) 2 Department of Biochemistry and Molecular Genetics, Faculty of Medicine, American University of Beirut, P.O. Box 11-0236 Riad El Solh, 1107 2020 Beirut, Lebanon; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +961-1-350-000 (ext. 4883). Received: 26 December 2012; in revised form: 22 January 2013 / Accepted: 1 February 2013 / Published: 1 March 2013 Abstract: The sphingolipid ceramide mediates various cellular processes in response to several extracellular stimuli. Some genotoxic stresses are able to induce p53-dependent ceramide accumulation leading to cell death. However, in other cases, in the absence of the tumor suppressor protein p53, apoptosis proceeds partly due to the activity of this “tumor suppressor lipid”, ceramide. In the current review, we describe ceramide and its roles in signaling pathways such as cell cycle arrest, hypoxia, hyperoxia, cell death, and cancer. In a specific manner, we are elaborating on the role of ceramide in mitochondrial apoptotic cell death signaling. -

Targeting Cancer Metabolism to Resensitize Chemotherapy: Potential Development of Cancer Chemosensitizers from Traditional Chinese Medicines
cancers Review Targeting Cancer Metabolism to Resensitize Chemotherapy: Potential Development of Cancer Chemosensitizers from Traditional Chinese Medicines Wei Guo, Hor-Yue Tan, Feiyu Chen, Ning Wang and Yibin Feng * School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 00000, China; [email protected] (W.G.); [email protected] (H.-Y.T.); [email protected] (F.C.); [email protected] (N.W.) * Correspondence: [email protected] Received: 22 December 2019; Accepted: 3 February 2020; Published: 10 February 2020 Abstract: Cancer is a common and complex disease with high incidence and mortality rates, which causes a severe public health problem worldwide. As one of the standard therapeutic approaches for cancer therapy, the prognosis and outcome of chemotherapy are still far from satisfactory due to the severe side effects and increasingly acquired resistance. The development of novel and effective treatment strategies to overcome chemoresistance is urgent for cancer therapy. Metabolic reprogramming is one of the hallmarks of cancer. Cancer cells could rewire metabolic pathways to facilitate tumorigenesis, tumor progression, and metastasis, as well as chemoresistance. The metabolic reprogramming may serve as a promising therapeutic strategy and rekindle the research enthusiasm for overcoming chemoresistance. This review focuses on emerging mechanisms underlying rewired metabolic pathways for cancer chemoresistance in terms of glucose and energy, lipid, amino acid, and nucleotide metabolisms, as well as other related metabolisms. In particular, we highlight the potential of traditional Chinese medicine as a chemosensitizer for cancer chemotherapy from the metabolic perspective. The perspectives of metabolic targeting to chemoresistance are also discussed. -

Abdominal Obesity and Cardiovascular Disease
Advances in Obesity Weight Management & Control Mini Review Open Access Abdominal obesity and cardiovascular disease Abstract Volume 3 Issue 2 - 2015 There is no doubt that obesity has become a major disease in modern times and it Rayan Saleh is definitely associated with cancer, neurodegeneration and heart disease. Scientific Department of Food and Nutritional Sciences, University of studies have resulted in a growing consensus on the way abdominal obesity is Reading, UK associated with inflammation and cardiometabolic risk. Although the gender is a substantial factor of having abdominal fat, there are other protective factors including Correspondence: Rayan Saleh, Registered Dietitian, healthy eating and physical activity. Several techniques are used to assess obesity Department of Food and Nutritional sciences, University of and their utilization depends on their feasibility and economic cost. This research is Reading, White knights, Reading, RG6 6AH, Berkshire, UK, designed to address the important relationship between abdominal obesity and the risk Email [email protected] of developing cardiovascular disease. Received: August 19, 2015 | Published: September 15, 2015 Keywords: abdominal obesity, metabolic syndrome, cardiovascular disease, body shape, inflammation, insulin resistance Abbreviations: WHO, world health organization; T2D, type to hip ratio WHR), bioelectrical impedance analysis (BIA), Dual 2 diabetes; BMI, body mass index; WC, waist circumference; WHR, energy X-ray absorptiometry (DXA), Computed tomography (CT) waist -

Správa O Činnosti Organizácie SAV Za Rok 2013
Ústav normálnej a patologickej fyziológie SAV Správa o činnosti organizácie SAV za rok 2013 Bratislava január 2014 Obsah osnovy Správy o činnosti organizácie SAV za rok 2013 1. Základné údaje o organizácii 2. Vedecká činnosť 3. Doktorandské štúdium, iná pedagogická činnosť a budovanie ľudských zdrojov pre vedu a techniku 4. Medzinárodná vedecká spolupráca 5. Vedná politika 6. Spolupráca s VŠ a inými subjektmi v oblasti vedy a techniky 7. Spolupráca s aplikačnou a hospodárskou sférou 8. Aktivity pre Národnú radu SR, vládu SR, ústredné orgány štátnej správy SR a iné organizácie 9. Vedecko-organizačné a popularizačné aktivity 10. Činnosť knižnično-informačného pracoviska 11. Aktivity v orgánoch SAV 12. Hospodárenie organizácie 13. Nadácie a fondy pri organizácii SAV 14. Iné významné činnosti organizácie SAV 15. Vyznamenania, ocenenia a ceny udelené pracovníkom organizácie SAV 16. Poskytovanie informácií v súlade so zákonom o slobodnom prístupe k informáciám 17. Problémy a podnety pre činnosť SAV PRÍLOHY A Zoznam zamestnancov a doktorandov organizácie k 31.12.2013 B Projekty riešené v organizácii C Publikačná činnosť organizácie D Údaje o pedagogickej činnosti organizácie E Medzinárodná mobilita organizácie Správa o činnosti organizácie SAV 1. Základné údaje o organizácii 1.1. Kontaktné údaje Názov: Ústav normálnej a patologickej fyziológie SAV Riaditeľ: RNDr. Oľga Pecháňová, DrSc. Zástupca riaditeľa: MUDr. Fedor Jagla, CSc. Vedecký tajomník: RNDr. Iveta Bernátová, DrSc. Predseda vedeckej rady: RNDr. Iveta Bernátová, DrSc. Členovia snemu SAV: MUDr. Fedor Jagla, CSc., MUDr. Igor Riečanský, PhD. Adresa: Sienkiewiczova 1, 813 71 Bratislava http://www.unpf.sav.sk Tel.: 02/32296063 Fax: E-mail: [email protected] Názvy a adresy detašovaných pracovísk: nie sú Vedúci detašovaných pracovísk: nie sú Typ organizácie: Rozpočtová od roku 1953 1.2. -

Acute and Chronic Complications
Uniwersytet Medyczny w Łodzi Medical University of Lodz https://publicum.umed.lodz.pl Higher Blood Glucose Variability is Associated with Increased Risk of Hypoglycemia Publikacja / Publication in Well or Poorly Controlled Type 1 or Type 2 Diabetes, Czupryniak Leszek, Borkowska Anna, Szymańska-Garbacz Elektra DOI wersji wydawcy / Published http://dx.doi.org/10.2337/db17-381-663 version DOI Adres publikacji w Repozytorium URL / Publication address in https://publicum.umed.lodz.pl/info/article/AML063ddbabfba14480a6e45b1d944e1ccd/ Repository Data opublikowania w Repozytorium 2020-08-31 / Deposited in Repository on Rodzaj licencji / Type of licence Other open licence Czupryniak Leszek, Borkowska Anna, Szymańska-Garbacz Elektra : Higher Blood Glucose Variability is Associated with Increased Risk of Hypoglycemia in Well or Cytuj tę wersję / Cite this version Poorly Controlled Type 1 or Type 2 Diabetes, Diabetes, vol. 66, no. Suppl. 1, 2017, pp. 103-104, DOI:10.2337/db17-381-663 COMPLICATIONS—HYPOGLYCEMIA COMPLICATIONS—HYPOGLYCEMIA an activating role of SAMSN1, L-triiodothyronine, IFNA4, JAK1 and mTORC1, and an inhibitory action of BDNF, POR, ESR1, CTNNB1 and ERG on the gene networks identified in our samples. Moderated Poster Discussion: Hypoglycemia—Novel Concepts Our study for the first time characterizes the transcriptional responses (Posters: 381-P to 386-P), see page 19. of the BBB compartment to recurrent hypoglycemia exposure and may help identify novel therapeutic targets to restore the impaired responses against 381‑P hypoglycemia in patients with type 1 diabetes. & Supported By: National Institutes of Health; JDRF Hypoglycemia‑Associated Autonomic Failure Is Associated with POSTERS Complications Coordinated miRNA‑mRNA Network Changes in the Ventromedial Acute and Chronic Hypothalamus & 383‑P RAHUL AGRAWAL, CASEY TAYLOR, ADRIANA VIEIRA-DE-ABREU, SIMON J. -
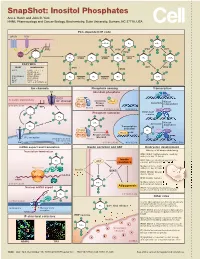
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Obesity and Reproduction: a Committee Opinion
Obesity and reproduction: a committee opinion Practice Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama The purpose of this ASRM Practice Committee report is to provide clinicians with principles and strategies for the evaluation and treatment of couples with infertility associated with obesity. This revised document replaces the Practice Committee document titled, ‘‘Obesity and reproduction: an educational bulletin,’’ last published in 2008 (Fertil Steril 2008;90:S21–9). (Fertil SterilÒ 2015;104:1116–26. Ó2015 Use your smartphone by American Society for Reproductive Medicine.) to scan this QR code Earn online CME credit related to this document at www.asrm.org/elearn and connect to the discussion forum for Discuss: You can discuss this article with its authors and with other ASRM members at http:// this article now.* fertstertforum.com/asrmpraccom-obesity-reproduction/ * Download a free QR code scanner by searching for “QR scanner” in your smartphone’s app store or app marketplace. he prevalence of obesity as a exceed $200 billion (7). This populations have a genetically higher worldwide epidemic has underestimates the economic burden percent body fat than Caucasians, T increased dramatically over the of obesity, since maternal morbidity resulting in greater risks of developing past two decades. In the United States and adverse perinatal outcomes add diabetes and CVD at a lower BMI of alone, almost two thirds of women additional costs. The problem of obesity 23–25 kg/m2 (12). and three fourths of men are overweight is also exacerbated by only one third of Known associations with metabolic or obese, as are nearly 50% of women of obese patients receiving advice from disease and death from CVD include reproductive age and 17% of their health-care providers regarding weight BMI (J-shaped association), increased children ages 2–19 years (1–3). -

PDF (Ph.D.Thesis)
The biological and clinical characterisation and validation of novel biomarkers in colorectal cancer Seán Fitzgerald B.Sc., M.Sc. This thesis is submitted to Dublin City University for the degree of Ph.D. July 2015 Based on research carried out at School of Biotechnology, Dublin City University, Dublin 9, Ireland. Supervisors: Professor Richard O’Kennedy Dr. Gregor Kijanka External Supervisor: Professor Elaine Kay Department of Pathology, RCSI, Beaumont Hospital. i Declaration I hereby certify that this material, which I now submit for assessment on the programme of study leading to the award of Ph.D. is entirely my own work, that I have exercised reasonable care to ensure that the work is original, and does not to the best of my knowledge breach any law of copyright, and has not been taken from the work of others save and to the extent that such work has been cited and acknowledged within the text of my work. Signed: ____________ ID No.: ___________ Date: _______ ii Acknowledgements Firstly, I would like to express my sincere gratitude and appreciation to my supervisors Prof. Richard O’Kennedy, Prof. Elaine Kay and Dr. Gregor Kijanka. This thesis would not have been possible without the expert advice and guidance that I received from each of you, both on an academic and personal level. I am especially grateful to Dr. Gregor Kijanka for his endless guidance, wisdom and friendship throughout my PhD. I would like to thank all the members of the Applied Biochemistry Group and the School of Biotechnology in DCU for their help, support and friendship over the last few years. -

Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt
International Journal of Molecular Sciences Article Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt 1,2, 1, 2 2 Stephanie Schwalm y, Martin Erhardt y, Isolde Römer , Josef Pfeilschifter , Uwe Zangemeister-Wittke 1,* and Andrea Huwiler 1,* 1 Institute of Pharmacology, University of Bern, Inselspital, INO-F, CH-3010 Bern, Switzerland; [email protected] (S.S.); [email protected] (M.E.) 2 Institute of General Pharmacology and Toxicology, University Hospital Frankfurt am Main, Goethe-University, Theodor-Stern Kai 7, D-60590 Frankfurt am Main, Germany; [email protected] (I.R.); [email protected] (J.P.) * Correspondence: [email protected] (U.Z.-W.); [email protected] (A.H.); Tel.: +41-31-6323214 (A.H.) These authors contributed equally to this work. y Received: 29 November 2019; Accepted: 12 February 2020; Published: 19 February 2020 Abstract: Ceramide kinase (CerK) is a lipid kinase that converts the proapoptotic ceramide to ceramide 1-phosphate, which has been proposed to have pro-malignant properties and regulate cell responses such as proliferation, migration, and inflammation. We used the parental human breast cancer cell line MDA-MB-231 and two single cell progenies derived from lung and bone metastasis upon injection of the parental cells into immuno-deficient mice. The lung and the bone metastatic cell lines showed a marked upregulation of CerK mRNA and activity when compared to the parental cell line. The metastatic cells also had increased migratory and invasive activity, which was dose-dependently reduced by the selective CerK inhibitor NVP-231. -

Vascular Metabolic Dysfunction and Lipotoxicity
Physiol. Res. 56: 149-158, 2007 Vascular Metabolic Dysfunction and Lipotoxicity H. M. MATTERN, C. D. HARDIN Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, MO, USA Received November 10, 2005 Accepted March 7, 2006 On-line available March 23, 2006 Summary The purpose of this study was to determine the role of lipotoxicity in vascular smooth muscle (VSM). C1-BODIPY 500/510 C12 used to assess the ability of VSM A7r5 cells to transport long-chain fatty acids showed that lipid transport did not appear to limit metabolism. Thin layer chromatography revealed that storage of transported fatty acid occurred primarily as mono- and diglycerides and fatty acids but not as triglycerides. We used lipid-induced apoptosis as a measure of lipotoxicity and found that 1.5 mM palmitate (6.8:1) bound to albumin resulted in a 15-fold increase in the number of apoptotic cells compared to the control at 24 hours. This apoptosis did not seem to be due to an increase in reactive oxygen species (ROS) since VSM cells incubated in palmitate showed less ROS production than cells incubated in albumin only. Similar exposure to oleate did not significantly increase the number of apoptotic cells compared to the control. Oleate actually significantly attenuated the apoptosis induced by palmitate, suggesting that unsaturated fatty acids have a protective effect on cells undergoing palmitate-induced apoptosis. These results suggest that vascular smooth muscle is vulnerable to lipotoxicity and that this lipotoxicity may play a role in the development of atherosclerosis. Key words Vascular smooth muscle • Palmitate • Oleate • Apoptosis • Reactive oxygen species Introduction Geng and Libby 2002). -

Skeletal Muscle Lipotoxicity in Insulin Resistance and Type 2 Diabetes: a Causal Mechanism Or an Innocent Bystander?
176:2 C Brøns and L G Grunnet Dysfunctional adipose tissue 176:2 R67–R78 Review and T2D MECHANISMSPROOF IN ENDOCRINOLOGY ONLY Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: a causal mechanism or an innocent bystander? Charlotte Brøns and Louise Groth Grunnet Correspondence should be addressed Department of Endocrinology (Diabetes and Metabolism), Rigshospitalet, Copenhagen, Denmark to C Brøns Email [email protected] Abstract Dysfunctional adipose tissue is associated with an increased risk of developing type 2 diabetes (T2D). One characteristic of a dysfunctional adipose tissue is the reduced expandability of the subcutaneous adipose tissue leading to ectopic storage of fat in organs and/or tissues involved in the pathogenesis of T2D that can cause lipotoxicity. Accumulation of lipids in the skeletal muscle is associated with insulin resistance, but the majority of previous studies do not prove any causality. Most studies agree that it is not the intramuscular lipids per se that causes insulin resistance, but rather lipid intermediates such as diacylglycerols, fatty acyl-CoAs and ceramides and that it is the localization, composition and turnover of these intermediates that play an important role in the development of insulin resistance and T2D. Adipose tissue is a more active tissue than previously thought, and future research should thus aim at examining the exact role of lipid composition, cellular localization and the dynamics of lipid turnover on the development of insulin resistance. In addition, ectopic storage of fat has differential impact on various organs in different phenotypes at risk of developing T2D; thus, understanding how adipogenesis is regulated, the interference European Journal European of Endocrinology with metabolic outcomes and what determines the capacity of adipose tissue expandability in distinct population groups is necessary. -

Inhibition of Spinach Phosphoribulokinase by DL-Glyceraldehyde by ANTONI R
Biochem. J. (1976) 153, 613-619 613 Printed in Great Britain Inhibition of Spinach Phosphoribulokinase by DL-Glyceraldehyde By ANTONI R. SLABAS and DAVID A. WALKER Department ofBotany, University ofSheffield, Sheffield S1O 2TN, U.K. (Received 10 September 1975) Spinach chloroplast phosphoribulokinase is inhibited by DL-glyceraldehyde. The in- hibition is non-competitive with respect to ribulose 5-phosphate (Ki 19mM) and ATP (Ki 20mM). The inhibition is discussed in relation to a previously reported inhibition of CO2 assimilation in intact and envelope-free chloroplasts by DL-glyceraldehyde. It is concluded that the inhibition of phosphoribulokinase is insufficient to account for the inhibition, by DL-glyceraldehyde, of 02 evolution with ribose 5-phosphate as substrate and that a further site of inhibition is also present in this system. DL-Glyceraldehyde inhibits photosynthetic carbon glycylglycine buffer, pH7.4, in a total volume of assiniilation by intact chloroplasts and by the re- 13 ml. The reaction was terminated by the addition constituted chloroplast system (Stokes & Walker, of 2ml of 50 % (w/v) trichloroacetic acid and the pH 1972). The inhibition is most pronounced with intact was adjusted to pH8.0 with KOH. After the addition chloroplasts, a concentration of 1OmM being sufficient of Sml of 1.OM-barium acetate the solution was to suppress 02 evolution completely. It is important centrifuged and the precipitate discarded after because DL-glyceraldehyde is the only known inhibi- washing with Sml of water. Cold ethanol (4vol.) was tor of photosynthesis that is entirely without detect- added to the combined supernatants (total volume able effect on photophosphorylation or photo- 25.4ml); the new precipitate was recovered by synthetic electron transport.