Implementation of a New Parcellation of the Orbitofrontal Cortex in the Automated Anatomical Labeling Atlas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -
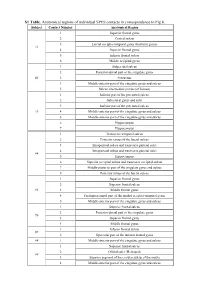
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
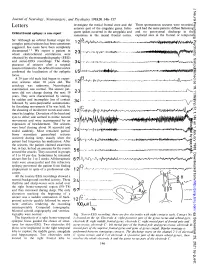
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -

Quantity Determination and the Distance Effect with Letters, Numbers, and Shapes: a Functional MR Imaging Study of Number Processing
AJNR Am J Neuroradiol 23:193–200, February 2003 Quantity Determination and the Distance Effect with Letters, Numbers, and Shapes: A Functional MR Imaging Study of Number Processing Robert K. Fulbright, Stephanie C. Manson, Pawel Skudlarski, Cheryl M. Lacadie, and John C. Gore BACKGROUND AND PURPOSE: The ability to quantify, or to determine magnitude, is an important part of number processing, and the extent to which language and other cognitive abilities are involved with number processing is an area of interest. We compared activation patterns, reaction times, and accuracy as subjects determined stimulus magnitude by ordering letters, numbers, and shapes. A second goal was to define the brain regions involved in the distance effect (the farther apart numbers are, the faster subjects are at judging which number is larger) and whether this effect depended on stimulus type. METHODS: Functional MR images were acquired in 19 healthy subjects. The order task required the subjects to judge whether three stimuli were in order according to their position in the alphabet (letters), position in the number line (numbers), or size (shapes). In the control (identify task), subjects judged whether one of the three stimuli was a particular letter, number, or shape. Each stimulus type was divided into near trials (quantity difference of three or less) and far trials (quantity difference of at least five) to assess the distance effect. RESULTS: Subjects were less accurate and slower with letters than with numbers and shapes. A distance effect was present with shapes and numbers, as subjects ordered the near trials slower than far trials. No distance effect was detected with letters. -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger).