Altered Resting State Connectivity of the Insular Cortex in Individuals with Fibromyalgia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -
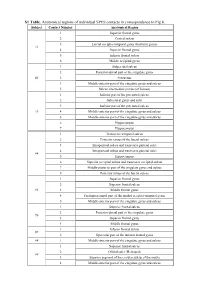
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology. -

Lucid Dreaming and the Prefrontal Cortex Performance III
Lucid Dreaming and the Prefrontal Cortex Performance III Georgia Minkoff and Grace Boyar Briarcliff High School Georgia Minkoff and Grace Boyar Briarcliff High School 2 First, we would like to thank our mentor, Dr. Peter Morgan, and Sarah Hodges from the Yale Medical Research Center for their immense support and guidance throughout our project. We would also like to extend a thank you to our teachers Michael Inglis and Annmarie O’Brien, for supervising us throughout our three years in Science Research and making them such an enlightening experience. Finally, we would like to thank all of the students that participated in our study, whose time and effort was greatly appreciated. Abstract Title: Lucid Dreaming and the Prefrontal Cortex III Name and address: Georgia Minkoff and Grace Boyar School/city/state: Briarcliff High School, Briarcliff Manor, NY 10510 Teacher: Mr. Michael Inglis and Ms. Annmarie O’Brien Mentor: Dr. Peter T. Morgan Scientific discipline: Neurological Science Objectives The purpose of this study is to prove that ventromedial but not the dorsolateral prefrontal cortical functions will prove one’s ability to lucid dream. The precuneus is also tested to distinguish lucid from non-lucid dreamers. Each part of the brain listed above is correlated to cognitive behaviors (i.e.; decision making, risk taking, reaction time, and memory). These functions are observed throughout the study to test their degree of enhancement. Methods Each participant endured a week of intense lucid dreaming induction treatment. They were administered questionnaires, assessments, cognitive computer tasks, and a dream journal. While the questionnaires were only completed once, computer tasks were done at the beginning and end of the study to compare the development of the behaviors tested. -

Revista Brasileira De Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012
Rev Bras Psiquiatr. 2012;34:101-111 Revista Brasileira de Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012 REVIEW ARTICLE Neuroimaging in specific phobia disorder: a systematic review of the literature Ila M.P. Linares,1 Clarissa Trzesniak,1 Marcos Hortes N. Chagas,1 Jaime E. C. Hallak,1 Antonio E. Nardi,2 José Alexandre S. Crippa1 ¹ Department of Neuroscience and Behavior of the Ribeirão Preto Medical School, Universidade de São Paulo (FMRP-USP). INCT Translational Medicine (CNPq). São Paulo, Brazil 2 Panic & Respiration Laboratory. Institute of Psychiatry, Universidade Federal do Rio de Janeiro (UFRJ). INCT Translational Medicine (CNPq). Rio de Janeiro, Brazil Received on August 03, 2011; accepted on October 12, 2011 DESCRIPTORS Abstract Neuroimaging; Objective: Specific phobia (SP) is characterized by irrational fear associated with avoidance of Specific Phobia; specific stimuli. In recent years, neuroimaging techniques have been used in an attempt to better Review; understand the neurobiology of anxiety disorders. The objective of this study was to perform a Anxiety Disorder; systematic review of articles that used neuroimaging techniques to study SP. Method: A literature Phobia. search was conducted through electronic databases, using the keywords: imaging, neuroimaging, PET, spectroscopy, functional magnetic resonance, structural magnetic resonance, SPECT, MRI, DTI, and tractography, combined with simple phobia and specific phobia. One-hundred fifteen articles were found, of which 38 were selected for the present review. From these, 24 used fMRI, 11 used PET, 1 used SPECT, 2 used structural MRI, and none used spectroscopy. Result: The search showed that studies in this area were published recently and that the neuroanatomic substrate of SP has not yet been consolidated. -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Microsurgical and Tractographic Anatomical Study of Insular and Transsylvian Transinsular Approach
Neurol Sci (2011) 32:865–874 DOI 10.1007/s10072-011-0721-2 ORIGINAL ARTICLE Microsurgical and tractographic anatomical study of insular and transsylvian transinsular approach Feng Wang • Tao Sun • XinGang Li • HeChun Xia • ZongZheng Li Received: 29 September 2008 / Accepted: 16 July 2011 / Published online: 24 August 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract This study is to define the operative anatomy of Introduction the insula with emphasis on the transsylvian transinsular approach. The anatomy was studied in 15 brain specimens, In humans, the insula is a highly developed structure, among five were dissected by use of fiber dissection totally encased within the brain. In many clinical and technique; diffusion tensor imaging of 10 healthy volun- experimental studies, a variety of functions have been teers was obtained with a 1.5-T MR system. The temporal attributed to the insula, however, the full and comprehen- stem consists mainly of the uncinate fasciculus, inferior sive role that it plays continues to remain obscure. Oper- occipitofrontal fasciculus, Meyer’s loop of the optic radi- ation of neurosurgery, specifically of epilepsy surgery, is a ation and anterior commissure. The transinsular approach window onto function and dysfunction of the human brain requires an incision of the inferior limiting sulcus. In this [1]. The insula, as part of the paralimbic system, has both procedure, the fibers of the temporal stem can be inter- invasive anatomical and functional connections with the rupted to various degrees. The fiber dissection technique is temporal lobe through white matter fibers [2–6]. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472