Resting-State Brain Functional Connectivity in Patients with Chronic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology. -

Lucid Dreaming and the Prefrontal Cortex Performance III
Lucid Dreaming and the Prefrontal Cortex Performance III Georgia Minkoff and Grace Boyar Briarcliff High School Georgia Minkoff and Grace Boyar Briarcliff High School 2 First, we would like to thank our mentor, Dr. Peter Morgan, and Sarah Hodges from the Yale Medical Research Center for their immense support and guidance throughout our project. We would also like to extend a thank you to our teachers Michael Inglis and Annmarie O’Brien, for supervising us throughout our three years in Science Research and making them such an enlightening experience. Finally, we would like to thank all of the students that participated in our study, whose time and effort was greatly appreciated. Abstract Title: Lucid Dreaming and the Prefrontal Cortex III Name and address: Georgia Minkoff and Grace Boyar School/city/state: Briarcliff High School, Briarcliff Manor, NY 10510 Teacher: Mr. Michael Inglis and Ms. Annmarie O’Brien Mentor: Dr. Peter T. Morgan Scientific discipline: Neurological Science Objectives The purpose of this study is to prove that ventromedial but not the dorsolateral prefrontal cortical functions will prove one’s ability to lucid dream. The precuneus is also tested to distinguish lucid from non-lucid dreamers. Each part of the brain listed above is correlated to cognitive behaviors (i.e.; decision making, risk taking, reaction time, and memory). These functions are observed throughout the study to test their degree of enhancement. Methods Each participant endured a week of intense lucid dreaming induction treatment. They were administered questionnaires, assessments, cognitive computer tasks, and a dream journal. While the questionnaires were only completed once, computer tasks were done at the beginning and end of the study to compare the development of the behaviors tested. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Altered Resting State Connectivity of the Insular Cortex in Individuals with Fibromyalgia
The Journal of Pain, Vol 15, No 8 (August), 2014: pp 815-826 Available online at www.jpain.org and www.sciencedirect.com Original Reports Altered Resting State Connectivity of the Insular Cortex in Individuals With Fibromyalgia Eric Ichesco,* Tobias Schmidt-Wilcke,*,** Rupal Bhavsar,*,y Daniel J. Clauw,* Scott J. Peltier,z Jieun Kim,x Vitaly Napadow,x,{,k Johnson P. Hampson,* Anson E. Kairys,*,yy David A. Williams,* and Richard E. Harris* *Department of Anesthesiology, Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan. yNeurology Department, University of Pennsylvania, Philadelphia, Pennsylvania. z Functional MRI Laboratory, University of Michigan, Ann Arbor, Michigan. xMGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts. {Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts. kDepartment of Radiology, Logan College of Chiropractic, Chesterfield, Missouri. **Department of Neurology, Bergmannsheil, Ruhr University Bochum, Bochum, Germany. yyDepartment of Psychology, University of Colorado Denver, Denver, Colorado. Abstract: The insular cortex (IC) and cingulate cortex (CC) are critically involved in pain perception. Previously we demonstrated that fibromyalgia (FM) patients have greater connectivity between the insula and default mode network at rest, and that changes in the degree of this connectivity were associated with changes in the intensity of ongoing clinical pain. In this study we more thoroughly evaluated the degree of resting-state connectivity to multiple regions of the IC in individuals with FM and healthy controls. We also investigated the relationship between connectivity, experimental pain, and current clinical chronic pain. Functional connectivity was assessed using resting-state functional magnetic resonance imaging in 18 FM patients and 18 age- and sex-matched healthy controls using predefined seed regions in the anterior, middle, and posterior IC. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -
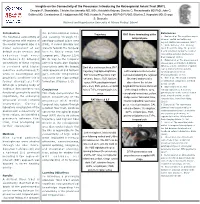
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT)
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT). Georgios P. Skandalakis; Christos Koutsarnakis MD, MSc; Aristotelis Kalyvas; Dimitris G. Placantonakis MD PhD; John G. Golfinos MD; Constantinos G. Hadjipanayis MD PhD; Kostas N. Fountas MD PhD FAANS; Eftychia Z. Kapsalaki MD; George S. Stranjalis National and Kapodistrian University of Athens Medical School Introduction the parieto-occipital sulcus, References Trajectory RAT fibers terminating at the The functional connectivity of and passing through the 1. Epstein et al. The cognitive map in temporal pole humans: spatial navigation and the precuneus with regions of parahippocampal place area beyond. Nature neuroscience. (2017) the medial temporal lobe is a (PPA), it curves laterally and 2. Wade-Bohleber et al. Thinking major component of our projects towards the temporal about the past to shape the present: default mode network and horn to finally reach the Neural activation during the recall of relationship episodes. Behavioural underlies high order temporal pole. (Figures 1,3,4) Brain Research (2018). functions.(1-5) Stronger On its way to the temporal 3. Hebscher et al. The precuneus and connectivity of these regions pole this tracts also displays hippocampus contribute to individual differences in the unfolding of spatial is corelated with higher connections with the lingual, Dark blue continuous lines, RAT representations during episodic cognitive performances(7) parahippocampal and fusiform trajectory; highlighted light blue, Left hemisphere infero-medial autobiographical memory. while in neurological and gyri, inferior longitudinal RAT Cortical Projections; CaF, view demonstrating the regional Neuropsychologia. (2018) psychiatric conditions this is fasciculus and hippocampal calcarine fissure; FuG, fusiform fiber tract anatomy after 4. -

A Scoping Review of Neuromodulation Techniques in Neurodegenerative Diseases: a Useful Tool for Clinical Practice?
medicina Review A Scoping Review of Neuromodulation Techniques in Neurodegenerative Diseases: A Useful Tool for Clinical Practice? Fabio Marson 1,2 , Stefano Lasaponara 3,4 and Marco Cavallo 5,6,* 1 Research Institute for Neuroscience, Education and Didactics, Fondazione Patrizio Paoletti, 06081 Assisi, Italy; [email protected] 2 Department of Human Neuroscience, Sapienza University of Rome, 00185 Rome, Italy 3 Department of Psychology, Sapienza University of Rome, 00185 Rome, Italy; [email protected] 4 Department of Human Sciences, LUMSA University, 00193 Rome, Italy 5 Faculty of Psychology, eCampus University, 22060 Novedrate, Italy 6 Clinical Psychology Service, Saint George Foundation, 12030 Cavallermaggiore, Italy * Correspondence: [email protected]; Tel.: +39-347-830-6430 Abstract: Background and Objectives: Neurodegenerative diseases that typically affect the elderly such as Alzheimer’s disease, Parkinson’s disease and frontotemporal dementia are typically char- acterised by significant cognitive impairment that worsens significantly over time. To date, viable pharmacological options for the cognitive symptoms in these clinical conditions are lacking. In recent years, various studies have employed neuromodulation techniques to try and contrast patients’ decay. Materials and Methods: We conducted an in-depth literature review of the state-of-the-art of the contribution of these techniques across these neurodegenerative diseases. Results: The present review reports that neuromodulation techniques targeting cognitive impairment do not allow to Citation: Marson, F.; Lasaponara, S.; Cavallo, M. A Scoping Review of draw yet any definitive conclusion about their clinical efficacy although preliminary evidence is very Neuromodulation Techniques in encouraging. Conclusions: Further and more robust studies should evaluate the potentialities and Neurodegenerative Diseases: A limitations of the application of these promising therapeutic tools to neurodegenerative diseases. -

Brain Regions Supporting Verbal Memory Improvement in Healthy Older Subjects Regiões Do Cérebro Relacionadas À Melhora Da Memória Verbal Em Idosos Saudáveis Eliane C
DOI: 10.1590/0004-282X20140120 ARTICLE Brain regions supporting verbal memory improvement in healthy older subjects Regiões do cérebro relacionadas à melhora da memória verbal em idosos saudáveis Eliane C. Miotto1, Joana B. Balardin1, Cary R. Savage2, Maria da Graça M. Martin3, Marcelo C. Batistuzzo1, Edson Amaro Junior3, Ricardo Nitrini1 ABSTRACT Despite growing interest in developing cognitive training interventions to minimize the aging cognitive decline process, no studies have attempted to explore which brain regions support the application of semantic strategies during verbal memory encoding. Our aim was to investigate the behavioral performance and brain correlates of these strategies in elderly individuals using fMRI in healthy older subjects. Method: Subjects were scanned twice on the same day, before and after, directed instructions to apply semantic strategies during the encoding of word lists. Results: Improved memory performance associated to increased semantic strategy application and brain activity in the left inferior and middle and right medial superior prefrontal cortex were found after the directed instructions. There was also reduced activation in areas related to strategy mobilization. Conclusion: Improved memory performance in older subjects after the application of semantic strategies was associated with functional brain reorganization involving regions inside and outside the typical memory network. Keywords: older subjects, semantic strategies, fMRI, episodic memory. RESUMO Apesar do crescente interesse em intervenções de treinamento cognitivo para minimizar o declínio cognitivo do envelhecimento, nenhum estudo explorou quais regiões do cérebro estão relacionadas à aplicação de estratégias semânticas durante a codificação da memória verbal. Nosso objetivo foi investigar o comportamento e correlatos cerebrais associados a essas estratégias usando fMRI em idosos saudáveis. -

Measurement of Precuneal and Hippocampal Volumes Using Magnetic Resonance Volumetry in Alzheimer’S Disease
ORIGINAL ARTICLE Print ISSN 1738-6586 / On-line ISSN 2005-5013 J Clin Neurol 2010;6:196-203 10.3988/jcn.2010.6.4.196 Measurement of Precuneal and Hippocampal Volumes Using Magnetic Resonance Volumetry in Alzheimer’s Disease Seon-Young Ryu, MD, PhDa; Min Jeong Kwon, PhDb; Sang-Bong Lee, MD, PhDa; Dong Won Yang, MD, PhDc; Tae-Woo Kim, MDa; In-Uk Song, MD, PhDd; Po Song Yang, MD, PhDb; Hyun Jeong Kim, MD, PhDb; Ae Young Lee, MD, PhDe aDepartments of Neurology and bRadiology, Daejeon St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Dajeon, Korea cDepartment of Neurology, Seoul St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea dDepartment of Neurology, Incheon St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Incheon, Korea eDepartment of Neurology, Chungnam National University Hospital, Daejeon, Korea Background and PurposezzAlzheimer’s disease (AD) is associated with structural alterations in the medial temporal lobe (MTL) and functional alterations in the posterior cortical region, es- pecially in the early stages. However, it is unclear what mechanisms underlie these regional discrep- ancies or whether the posterior cortical hypometabolism reflects disconnection from the MTL le- sion or is the result of local pathology. The precuneus, an area of the posteromedial cortex that is involved in the early stages of AD, has recently received a great deal of attention in functional neu- roimaging studies. To assess the relationship between the precuneus and hippocampus in AD, we investigated the volumes of these two areas using a magnetic resonance volumetric method. -

Functional Connectivity of the Insula in the Resting Brain
NeuroImage 55 (2011) 8–23 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Functional connectivity of the insula in the resting brain Franco Cauda a,b,⁎, Federico D'Agata a,b,c, Katiuscia Sacco a,b, Sergio Duca a, Giuliano Geminiani a,b, Alessandro Vercelli d a CCS fMRI, Koelliker Hospital, Turin, Italy b Department of Psychology, University of Turin, Turin, Italy c Department of Neuroscience, AOU San Giovanni Battista, Turin, Italy d Department of Anatomy, Pharmacology and Forensic Medicine, National Institute of Neuroscience, Turin, Italy article info abstract Article history: The human insula is hidden in the depth of the cerebral hemisphere by the overlying frontal and temporal Received 4 June 2010 opercula, and consists of three cytoarchitectonically distinct regions: the anterior agranular area, posterior Revised 10 November 2010 granular area, and the transitional dysgranular zone; each has distinct histochemical staining patterns and Accepted 15 November 2010 specific connectivity. Even though there are several studies reporting the functional connectivity of the insula Available online 24 November 2010 with the cingulated cortex, its relationships with other brain areas remain elusive in humans. Therefore, we Keywords: decided to use resting state functional connectivity to elucidate in details its connectivity, in terms of cortical fl Default networks and subcortical areas, and also of lateralization. We investigated correlations in BOLD uctuations between Cingulate cortex specific