Supplementary Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -
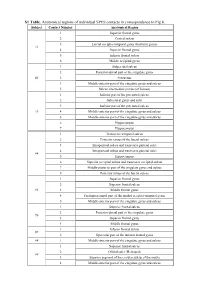
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation
r Human Brain Mapping 31:1316–1326 (2010) r The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation Pascal Molenberghs,* Christopher Brander, Jason B. Mattingley, and Ross Cunnington The University of Queensland, Queensland Brain Institute & School of Psychology, St Lucia, Queensland, Australia r r Abstract: It has been suggested that in humans the mirror neuron system provides a neural substrate for imitation behaviour, but the relative contributions of different brain regions to the imitation of manual actions is still a matter of debate. To investigate the role of the mirror neuron system in imita- tion we used fMRI to examine patterns of neural activity under four different conditions: passive ob- servation of a pantomimed action (e.g., hammering a nail); (2) imitation of an observed action; (3) execution of an action in response to a word cue; and (4) self-selected execution of an action. A net- work of cortical areas, including the left supramarginal gyrus, left superior parietal lobule, left dorsal premotor area and bilateral superior temporal sulcus (STS), was significantly active across all four con- ditions. Crucially, within this network the STS bilaterally was the only region in which activity was significantly greater for action imitation than for the passive observation and execution conditions. We suggest that the role of the STS in imitation is not merely to passively register observed biological motion, but rather to actively represent visuomotor correspondences between one’s own actions and the actions of others. Hum Brain Mapp 31:1316–1326, 2010. VC 2010 Wiley-Liss, Inc. Key words: fMRI; imitation; mirror neuron system r r INTRODUCTION ror neurons are visuomotor neurons that fire both when an action is performed and when a similar or identical Motor imitation involves observing the action of another action is passively observed [Rizzolatti and Craighero, individual and matching one’s own movements to those 2004]. -

Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia (Positron-Emission Tomography͞human͞brain͞regional͞cerebral)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8939–8944, July 1998 Neurobiology Functional connectivity of the angular gyrus in normal reading and dyslexia (positron-emission tomographyyhumanybrainyregionalycerebral) B. HORWITZ*†,J.M.RUMSEY‡, AND B. C. DONOHUE‡ *Laboratory of Neurosciences, National Institute on Aging, and ‡Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Robert H. Wurtz, National Eye Institute, Bethesda, MD, May 14, 1998 (received for review January 19, 1998) ABSTRACT The classic neurologic model for reading, not functionally connected during a specific task. On the other based on studies of patients with acquired alexia, hypothesizes hand, if rCBF in two regions is correlated, these regions need functional linkages between the angular gyrus in the left not be anatomically linked; their activities may be correlated, hemisphere and visual association areas in the occipital and for example, because both receive inputs from a third area (for temporal lobes. The angular gyrus also is thought to have more discussion about these connectivity concepts, see refs. 8, functional links with posterior language areas (e.g., Wer- 10, and 11). nicke’s area), because it is presumed to be involved in mapping Based on lesion studies in many patients with alexia, it has visually presented inputs onto linguistic representations. Us- been proposed that the posterior portion of the neural network ing positron emission tomography , we demonstrate in normal mediating reading in the left cerebral hemisphere involves men that regional cerebral blood flow in the left angular gyrus functional links between the angular gyrus and extrastriate shows strong within-task, across-subjects correlations (i.e., areas in occipital and temporal cortex associated with the functional connectivity) with regional cerebral blood flow in visual processing of letter and word-like stimuli (12–14). -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Autism and the Social Brain a Visual Tour
Autism and the Social Brain A Visual Tour Charles Cartwright, M.D. Director YAI Autism Center “Social cognition refers to the fundamental abilities to perceive, categorize, remember, analyze, reason with, and behave toward other conspecifics” Pelphrey and Carter, 2008 Abbreviation Structure AMY Amygdala EBA Extrastriate body area STS Superior temporal sulcus TPJ Temporal parietal junction FFG Fusiform gyrus OFC Orbital frontal gyrus mPFC Medial prefrontal cortex IFG Inferior frontal Pelphrey and Carter 2008 gyrus Building Upon Structures and Function Pelphrey and Carter 2008 Neuroimaging technologies • Explosion of neuroimaging technology – Faster, more powerful magnets – Better resolution images showing thinner slices of brain • Visualize the brain in action • Explore relationship between brain structure, function, and human behavior. • Help identify what changes unfold in brain disorders like autism Neuroimaging in ASDs • No reliable neuroimaging marker • Imaging not recommended as part of routine work- up • Neuroimaging research can provide clues to brain structure and function and to neurodevelopmental origins Structural Brain Imaging • MRI and CT • Allows for examination of structure of the brain • Identifies abnormalities in different areas of brain • Helps understand patterns of brain development over time MRI • Magnetic Resonance Imaging • Uses magnetic fields and radio waves to produce high-quality two- or three dimensional images of brain structures • Large cylindrical magnet creates magnetic field around head; radio waves are -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology. -

Lucid Dreaming and the Prefrontal Cortex Performance III
Lucid Dreaming and the Prefrontal Cortex Performance III Georgia Minkoff and Grace Boyar Briarcliff High School Georgia Minkoff and Grace Boyar Briarcliff High School 2 First, we would like to thank our mentor, Dr. Peter Morgan, and Sarah Hodges from the Yale Medical Research Center for their immense support and guidance throughout our project. We would also like to extend a thank you to our teachers Michael Inglis and Annmarie O’Brien, for supervising us throughout our three years in Science Research and making them such an enlightening experience. Finally, we would like to thank all of the students that participated in our study, whose time and effort was greatly appreciated. Abstract Title: Lucid Dreaming and the Prefrontal Cortex III Name and address: Georgia Minkoff and Grace Boyar School/city/state: Briarcliff High School, Briarcliff Manor, NY 10510 Teacher: Mr. Michael Inglis and Ms. Annmarie O’Brien Mentor: Dr. Peter T. Morgan Scientific discipline: Neurological Science Objectives The purpose of this study is to prove that ventromedial but not the dorsolateral prefrontal cortical functions will prove one’s ability to lucid dream. The precuneus is also tested to distinguish lucid from non-lucid dreamers. Each part of the brain listed above is correlated to cognitive behaviors (i.e.; decision making, risk taking, reaction time, and memory). These functions are observed throughout the study to test their degree of enhancement. Methods Each participant endured a week of intense lucid dreaming induction treatment. They were administered questionnaires, assessments, cognitive computer tasks, and a dream journal. While the questionnaires were only completed once, computer tasks were done at the beginning and end of the study to compare the development of the behaviors tested. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura
Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura To cite this version: Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura. Surgical Anatomy of the Insula. Mehmet Turgut; Canan Yurttaş; R. Shane Tubbs. Island of Reil (Insula) in the Human Brain. Anatomical, Functional, Clinical and Surgical Aspects, 19, Springer, pp.23-37, 2018, 978-3-319-75467-3. 10.1007/978-3-319-75468-0_3. hal-02539550 HAL Id: hal-02539550 https://hal.archives-ouvertes.fr/hal-02539550 Submitted on 15 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Surgical Anatomy of the insula Christophe Destrieux1, 2, Igor Lima Maldonado3, Louis-Marie Terrier1, 2, Ilyess Zemmoura1, 2 1 : Université François-Rabelais de Tours, Inserm, Imagerie et cerveau UMR 930, Tours, France 2 : CHRU de Tours, Service de Neurochirurgie , Tours, France 3 : Universidade Federal da Bahia, Laboratório de Anatomia e Dissecção, Departamento de Biomorfologia - Instituto de Ciências da Saúde, Salvador-Bahia, Brazil 1. Abstract The insula was for a long time considered as one of the most challenging areas of the brain. This is mainly related to its location, deep and medial to the fronto-parietal, temporal and fronto-orbital opercula.