Evidence of Cortical Thickness Reduction and Disconnection in High
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

(Mpfc-Lpmc) Affects Subjective Beauty but Not Ugliness
ORIGINAL RESEARCH published: 08 December 2015 doi: 10.3389/fnhum.2015.00654 Transcranial Direct Current Stimulation Over the Medial Prefrontal Cortex and Left Primary Motor Cortex (mPFC-lPMC) Affects Subjective Beauty but not Ugliness Koyo Nakamura1 and Hideaki Kawabata2* 1 Graduate School of Human Relations, Keio University, Tokyo, Japan, 2 Department of Psychology, Keio University, Tokyo, Japan Neuroaesthetics has been searching for the neural bases of the subjective experience of beauty. It has been demonstrated that neural activities in the medial prefrontal cortex (mPFC) and the left primary motor cortex (lPMC) correlate with the subjective experience of beauty. Although beauty and ugliness seem to be semantically and conceptually opposite, it is still unknown whether these two evaluations represent extreme opposites in unitary or bivariate dimensions. In this study, we applied transcranial direct current stimulation (tDCS) to examine whether non-invasive brain stimulation modulates two types of esthetic evaluation; evaluating beauty and ugliness. Participants rated the Edited by: subjective beauty and ugliness of abstract paintings before and after the application of Edward A. Vessel, tDCS. Application of cathodal tDCS over the mPFC with anode electrode over the lPMC, New York University, USA which induced temporal inhibition of neural excitability of the mPFC, led to a decrease Reviewed by: Zaira Cattaneo, in beauty ratings but not ugliness ratings. There were no changes in ratings of both University of Milano-Bicocca, Italy beauty and ugliness when applying anodal tDCS or sham stimulation over the mPFC. Andrea Antal, Results from our experiment indicate that the mPFC and the lPMC have a causal role University Medical Center Goettingen, Germany in generating the subjective experience of beauty, with beauty and ugliness evaluations Gerald Cupchik, constituting two distinct dimensions. -

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography
3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography Julien Dauguet1, Sharon Peled2, Vladimir Berezovskii3, Thierry Delzescaux4, Simon K. Warfield1, Richard Born3, and Carl-Fredrik Westin5 1 Computational Radiology Laboratory, Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2 Harvard Center for Neurodegeneration and Repair, Boston, USA 3 Department of Neurobiology, Harvard Medical School, Boston, USA 4 Service Hospitalier Fr´ed´eric Joliot, CEA, Orsay, France 5 Laboratory of Mathematics in Imaging, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA Abstract. A classical neural tract tracer, WGA-HRP, was injected at multiple sites within the brain of a macaque monkey. Histological sections of the labeled fiber tracts were reconstructed in 3D, and the fibers were segmented and registered with the anatomical post-mortem MRI from the same animal. Fiber tracing along the same pathways was performed on the DTI data using a classical diffusion tracing technique. The fibers derived from the DTI were compared with those segmented from the histology in order to evaluate the performance of DTI fiber tracing. While there was generally good agreement between the two methods, our results reveal certain limitations of DTI tractography, particularly at regions of fiber tract crossing or bifurcation. 1 Introduction Tracing neural connections lies at the heart of neuroanatomy, and has profound implications for the study of neural function and for the study of developmental and adult plasticity of the nervous system. It is also important for the study of neurodegenerative diseases, and for the planning of neurosurgical procedures, such as tumor ablation. -

Visual Cortex in Humans 251
Author's personal copy Visual Cortex in Humans 251 Visual Cortex in Humans B A Wandell, S O Dumoulin, and A A Brewer, using fMRI, and we discuss the main features of the Stanford University, Stanford, CA, USA V1 map. We then summarize the positions and proper- ties of ten additional visual field maps. This represents ã 2009 Elsevier Ltd. All rights reserved. our current understanding of human visual field maps, although this remains an active field of investigation, with more maps likely to be discovered. Finally, we Human visua l cortex comprises 4–6 billion neurons that are organ ized into more than a dozen distinct describe theories about the functional purpose and functional areas. These areas include the gray matter organizing principles of these maps. in the occi pital lobe and extend into the temporal and parietal lobes . The locations of these areas in the The Size and Location of Human Visual intact human cortex can be identified by measuring Cortex visual field maps. The neurons within these areas have a variety of different stimulus response proper- The entirety of human cortex occupies a surface area 2 ties. We descr ibe how to measure these visual field on the order of 1000 cm and ranges between 2 and maps, their locations, and their overall organization. 4 mm in thickness. Each cubic millimeter of cortex contains approximately 50 000 neurons so that neo- We then consider how information about patterns, objects, color s, and motion is analyzed and repre- cortex in the two hemispheres contain on the order of sented in these maps. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia (Positron-Emission Tomography͞human͞brain͞regional͞cerebral)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8939–8944, July 1998 Neurobiology Functional connectivity of the angular gyrus in normal reading and dyslexia (positron-emission tomographyyhumanybrainyregionalycerebral) B. HORWITZ*†,J.M.RUMSEY‡, AND B. C. DONOHUE‡ *Laboratory of Neurosciences, National Institute on Aging, and ‡Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Robert H. Wurtz, National Eye Institute, Bethesda, MD, May 14, 1998 (received for review January 19, 1998) ABSTRACT The classic neurologic model for reading, not functionally connected during a specific task. On the other based on studies of patients with acquired alexia, hypothesizes hand, if rCBF in two regions is correlated, these regions need functional linkages between the angular gyrus in the left not be anatomically linked; their activities may be correlated, hemisphere and visual association areas in the occipital and for example, because both receive inputs from a third area (for temporal lobes. The angular gyrus also is thought to have more discussion about these connectivity concepts, see refs. 8, functional links with posterior language areas (e.g., Wer- 10, and 11). nicke’s area), because it is presumed to be involved in mapping Based on lesion studies in many patients with alexia, it has visually presented inputs onto linguistic representations. Us- been proposed that the posterior portion of the neural network ing positron emission tomography , we demonstrate in normal mediating reading in the left cerebral hemisphere involves men that regional cerebral blood flow in the left angular gyrus functional links between the angular gyrus and extrastriate shows strong within-task, across-subjects correlations (i.e., areas in occipital and temporal cortex associated with the functional connectivity) with regional cerebral blood flow in visual processing of letter and word-like stimuli (12–14). -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

Autism and the Social Brain a Visual Tour
Autism and the Social Brain A Visual Tour Charles Cartwright, M.D. Director YAI Autism Center “Social cognition refers to the fundamental abilities to perceive, categorize, remember, analyze, reason with, and behave toward other conspecifics” Pelphrey and Carter, 2008 Abbreviation Structure AMY Amygdala EBA Extrastriate body area STS Superior temporal sulcus TPJ Temporal parietal junction FFG Fusiform gyrus OFC Orbital frontal gyrus mPFC Medial prefrontal cortex IFG Inferior frontal Pelphrey and Carter 2008 gyrus Building Upon Structures and Function Pelphrey and Carter 2008 Neuroimaging technologies • Explosion of neuroimaging technology – Faster, more powerful magnets – Better resolution images showing thinner slices of brain • Visualize the brain in action • Explore relationship between brain structure, function, and human behavior. • Help identify what changes unfold in brain disorders like autism Neuroimaging in ASDs • No reliable neuroimaging marker • Imaging not recommended as part of routine work- up • Neuroimaging research can provide clues to brain structure and function and to neurodevelopmental origins Structural Brain Imaging • MRI and CT • Allows for examination of structure of the brain • Identifies abnormalities in different areas of brain • Helps understand patterns of brain development over time MRI • Magnetic Resonance Imaging • Uses magnetic fields and radio waves to produce high-quality two- or three dimensional images of brain structures • Large cylindrical magnet creates magnetic field around head; radio waves are -
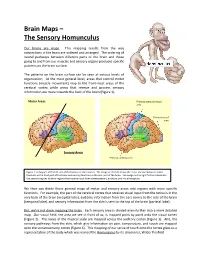
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Lesions in the Right Rolandic Operculum Are Associated with Self
www.nature.com/scientificreports OPEN Lesions in the right Rolandic operculum are associated with self‑rating afective and apathetic depressive symptoms for post‑stroke patients Stephanie Sutoko1,2*, Hirokazu Atsumori1,2, Akiko Obata1,2, Tsukasa Funane1,2, Akihiko Kandori1,2, Koji Shimonaga3,4, Seiji Hama2,4, Shigeto Yamawaki5 & Toshio Tsuji6 Stroke survivors majorly sufered from post‑stroke depression (PSD). The PSD diagnosis is commonly performed based on the clinical cut‑of for psychometric inventories. However, we hypothesized that PSD involves spectrum symptoms (e.g., apathy, depression, anxiety, and stress domains) and severity levels. Therefore, instead of using the clinical cut‑of, we suggested a data‑driven analysis to interpret patient spectrum conditions. The patients’ psychological conditions were categorized in an unsupervised manner using the k‑means clustering method, and the relationships between psychological conditions and quantitative lesion degrees were evaluated. This study involved one hundred sixty‑fve patient data; all patients were able to understand and perform self‑rating psychological conditions (i.e., no aphasia). Four severity levels—low, low‑to‑moderate, moderate‑ to‑high, and high—were observed for each combination of two psychological domains. Patients with worse conditions showed the signifcantly greater lesion degree at the right Rolandic operculum (part of Brodmann area 43). The dissimilarities between stress and other domains were also suggested. Patients with high stress were specifcally associated with lesions in the left thalamus. Impaired emotion processing and stress‑afected functions have been frequently related to those lesion regions. Those lesions were also robust and localized, suggesting the possibility of an objective for predicting psychological conditions from brain lesions.