Practical Cover Page(New2)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11-FLOWER DIAGRAMES, FORMULAS and FLOWER SYMETRY FLOWER FORMULAS and DIAGRAMES
11-FLOWER DIAGRAMES, FORMULAS AND FLOWER SYMETRY FLOWER FORMULAS and DIAGRAMES 1. FLOWER FORMULAS Floral formula is a means to represent the structure of a flower using numbers, letters and various symbols, presenting substantial information about the flower in a compact form. It can represent particular species, or can be generalized to characterize higher taxa, usually giving ranges of organ numbers. Floral formulae are one of the two ways of describing flower structure developed during the 19th century, the other being floral diagrams. Apart from the graphical diagrams, the flower structure can be characterized by textual formulae. A floral formula consists of five symbols indicating from left to right: Floral Symmetry Number of Tepal Number of Sepals Number of Petals Number of Stamens Number of Carpels Tepals Sepals Patals Stamen Carpels P K C A G The parts of the flower are described according to their arrangement from the outside to the inside of the flower. If an organ type is arranged in more whorls, the outermost is denoted first, and the whorls are separated by “+”. If the organ number is large or fluctuating, is denoted as “∞”. 2. FLOWER DIAGRAMES Floral diagram is a graphic representation of flower structure. It shows the number of floral organs, their arrangement and fusion. Different parts of the flower are represented by their respective symbols. Rather like floral formulas, floral diagrams are used to show symmetry, numbers of parts, the relationships of the parts to one another, and degree of connation and/or adnation. Such diagrams cannot easily show ovary position. FLOWER SYMMETRY Floral symmetry describes whether, and how, a flower in particular its perianth, can be divided into two or more identical or mirror-image parts. -

Basic Botany
Basic Botany - Flower Structure The Birmingham Botanical Gardens & Glasshouses Brief Descriptions of Activities Flower Structure • a Study Centre-led activity • Using large-scale models and bee (glove puppet) to take pupils through the basic flower parts and their functions Investigating Floral Structure A wide range of flowers are always on display in the glasshouses. Their structure can be recorded in a variety of ways: • Directed observation through use of questionnaires • Drawing half a flower and labelling its structure • Creating a plan of the flower as if viewed from above • Creating a simple floral formula (this worksheet is using a simplified form of the recording system used by botanists) See worksheets 1-4 at back of booklet. Pollination Mechanisms • An extension of this work is to look at a variety of ways in which plants are designed in order to attract different pollinators See ‘A Guide To Pollinators’ at back of booklet. • Busy Bees. This is a game where pupils act out pollination See worksheet 5 at back of booklet Guide To Pollinators “Bee Flowers” Typically yellow, blue or purple. They produce pollen and lots of nectar, are often marked with lines and blotches and are sweetly scented at certain times of the day. “Butterfly Flowers” Vivid colours, often purple, red or white. Usually open during the day with a long thin corolla tube, lots of nectar and a strong scent. “Moth Flowers” Often white, p ink or pale yellow, open at night and have a heavy scent. “Wasp Flowers” Often pinkish or dirty red, with horizontal or drooping cups into which the short tongued wasp can push its head. -
Sample Chapter
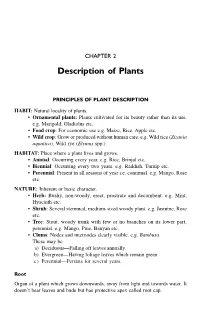
CHAPTER 2 Description of Plants PRINCIPLES OF PLANT DESCRIPTION HABIT: Natural locality of plants. • Ornamental plants: Plants cultivated for its beauty rather than its use. e.g. Marigold, Gladiolus etc. • Food crop: For economic use e.g. Maize, Rice, Apple etc. • Wild crop: Grow or produced without human care. e.g. Wild rice (Zizania aquatica), Wild rye (Elymus spp.). HABITAT: Place where a plant lives and grows. • Annual: Occurring every year. e.g. Rice, Brinjal etc. • Biennial: Occurring every two years. e.g. Raddish, Turnip etc. • Perennial: Present in all seasons of year i.e. continual. e.g. Mango, Rose etc. NATURE: Inherent or basic character. • Herb: Bushy, non-woody, erect, prostrate and decumbent. e.g. Mint, Hyacinth etc. • Shrub: Several stemmed, medium-sized woody plant. e.g. Jasmine, Rose etc. • Tree: Stout, woody trunk with few or no branches on its lower part, perennial. e.g. Mango, Pine, Banyan etc. • Clums: Nodes and internodes clearly visible. e.g. Bambusa These may be a) Deciduous—Falling off leaves annually. b) Evergreen—Having foliage leaves which remain green. c) Perennial—Persists for several years. Root Organ of a plant which grows downwards, away from light and towards water. It doesn’t bear leaves and buds but has protective apex called root cap. 10 Introduction to Pharmacognosy • Assimilatory root: Roots become green and serve for photosynthesis. e.g. Trapa • Tuberous root: Swollen, root without any definite shape. e.g. Sweet potato • Fasciculated root: Several tuberous roots occur in cluster at the base of stem. e.g. Dahlia • Nodulous root: Tuberous root becomes suddenly swollen at apex. -

Appendix 1 Vernacular Names
Appendix 1 Vernacular Names The vernacular names listed below have been collected from the literature. Few have phonetic spellings. Spelling is not helped by the difficulties of transcribing unwritten languages into European syllables and Roman script. Some languages have several names for the same species. Further complications arise from the various dialects and corruptions within a language, and use of names borrowed from other languages. Where the people are bilingual the person recording the name may fail to check which language it comes from. For example, in northern Sahel where Arabic is the lingua franca, the recorded names, supposedly Arabic, include a number from local languages. Sometimes the same name may be used for several species. For example, kiri is the Susu name for both Adansonia digitata and Drypetes afzelii. There is nothing unusual about such complications. For example, Grigson (1955) cites 52 English synonyms for the common dandelion (Taraxacum officinale) in the British Isles, and also mentions several examples of the same vernacular name applying to different species. Even Theophrastus in c. 300 BC complained that there were three plants called strykhnos, which were edible, soporific or hallucinogenic (Hort 1916). Languages and history are linked and it is hoped that understanding how lan- guages spread will lead to the discovery of the historical origins of some of the vernacular names for the baobab. The classification followed here is that of Gordon (2005) updated and edited by Blench (2005, personal communication). Alternative family names are shown in square brackets, dialects in parenthesis. Superscript Arabic numbers refer to references to the vernacular names; Roman numbers refer to further information in Section 4. -

Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected]
Course - B.Sc. Botany Semester - II Paper Code - BOT GE202 Paper Name – Plant Ecology and Taxonomy Topic - Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected] Bentham and Hooker Classification George Bentham (1800 – 1884) Joseph Hooker (1817 – 1911) It was Proposed by George Bentham ( 1800 – 1884 ) and Joseph Dalton Hooker (1817 – 1911 ) in their Genera Plantarum published during July (1862 ) & April ( 1883 ) George Bentham (1800 – 1884) and Joseph Dalton Hooker ( 1817 ) – 1911) , the two British botanist who were associated with the Royal Botanic garden at kew ( England) gave most important and easily workable system of classification of angiosperms and published it in three volume of ‘Genera plantarum ‘ The first part of Genera plantarum appeared in July 1882 and the last part in April 1883 . This was the greatest taxonomic work ever produced in the united kingdom and ever since been an inspiration to generations of kew botanists . Although Bentham and Hooker’s system of classification was based on that of A.P. de Candolle but greater stress was given on contrast between free and fused petals . Their symptom was widely accepted in Britain and commonwealth countries but in Europe and North America it did not hold the much ground . Bentham and Hooker divided the seed plants into Dicotyledons, Gymnosperms and Monocotyledons. They placed Ranales in beginning and grasses at the end . The following is the summary of Bentham & Hooker’s system. DICOTYLEDONS : A . Polypetalae ( petals are free ) Series l Thalamiflorae Order 1. Ranales eg. Ranunculaceae, Magnollaceae e.t.c Order 2. Parietales eg. Papaveraceae , Cruciferae e.t.c Order 3. -

POLYPETALAE – Petals Separate THALAMIFLORAE – Sepals, Petals and Stamens All Attached to Receptacle
POLYPETALAE – petals separate THALAMIFLORAE – Sepals, Petals and Stamens all attached to receptacle. Gynoecium apocarpous. RANUNCULACEAE (Herbaceous, leaves 3-parted) BERBERIDACEAE* (Carpel solitary, Anthers with flaps). Parietal placentation. [NOT Natural. Convergent evolution: Papaveraceae close to Ranunc., but remainder scattered amongst Rosids] PAPAVERACEAE* (Sepals 2, petals 4) CRUCIFERAE* (Petals 4, Stamens 6, ovary 2) CAPPARACEAE* (Ovary stalked) RESEDACEAE (Ovary open, 3-parted) CISTACEAE VIOLACEAE Ovary 2-3 septate. PITTOSPORACEAE* POLYGALACEAE. Axile placentation. CARYOPHYLLACEAE* PORTULACACEAE (Sepals gland-fringed) Stamens numerous; Calyx imbricate. GUTTIFERAE/CLUSIACEAE* THEACEAE Stamens numerous; Calyx valvate. MALVACEAE* (Anthers 1-celled) STERCULIACEAE TILIACEAE. DISCIFLORAE – Ovary superior, immersed in disk of flower. Ovule pendulous, raphe ventral;multiple series of stamens. LINACEAE; GERANIACEAE*; RUTACEAE Ovule pendulous, raphe dorsal OLACACEAE; AQUIFOLIACEAE*. Ovule erect, raphe ventral CELASTRACEAE; RHAMNACEAE*; VITACEAE. Ovule ascending, raphe ventral to reversed SAPINDACEAE*; ANACARDIACEAE CALYCIFLORAE – Stamens fused to Calyx of flower Ovaries separate, rarely united LEGUMINOSAE ROSACEAE* [SAXIFRAGALES. Carpels ±fused, separate styles: SAXIFRAGACEAE* (2 carps) CRASSULACEAE (5-6 carps) HAMAMELIDACEAE (2)] [: HYDRANGEACEAE – opp leaves, syncarpous. ESCALLONIACEAE – alt leaves dry pod => ASTERIDS Ovary syncarpous; divided into locules. MYRTACEAE (stamens numerous) LYTHRACEAE* ONAGRACEAE*. Ovary syncarpous; Parietal placentation LOASACEAE; TURNERACEAE; PASSIFLORACEAE; CUCURBITACEAE*; BEGONIACEAE; DATISCACEAE. Ficoidales – Ovary syncarpous; sub-basal placentation [the basal placentation is critical in placing these families among the Caryophyllids see above] CACTACEAE; AIZOACEAE.] Umbellales – Ovary syncarpous; 1 ovule per locule. [these families belong amongst the basal Asterids. Inferior ovaries but with separate petals] UMBELLIFERAE (2-locules); ARALIACEAE (5-locules); CORNACEAE. . -

Phytosociological Study of Coastal Flora of Devbhoomi Dwarka District and Its Islands in the Gulf of Kachchh, Gujarat
International Journal of Scientific Research in _______________________________ Research Paper . Biological Sciences Vol.6, Issue.3, pp.01-13, June (2019) E-ISSN: 2347-7520 DOI: https://doi.org/10.26438/ijsrbs/v6i3.113 Phytosociological study of coastal flora of Devbhoomi Dwarka district and its islands in the Gulf of Kachchh, Gujarat L. Das1*, H. Salvi2, R. D. Kamboj 3 1,3Gujarat Ecological Education and Research Foundation, Gandhinagar, Gujarat, India 2Department of Botany, Songadh Government Science College, Tapi, Gujarat, India *Corresponding Author: [email protected]; Tel.: +91-7573020436 Available online at: www.isroset.org Received: 16/May/2019, Accepted: 02/Jun/2019, Online: 30/Jun/2019 Abstract- The study described the diversity and phytosociological attributes of plant species (trees, shrubs and herbs) in coastal areas of Devbhoomi Dwarka District and its islands in the Gulf of Kachchh. A random sampling method was employed in this study. A total of 243 plant species were recorded of which trees and shrubs represented with 30 specieseach. Grasses & sedges were also represented by 30 species and 29 species were climbers. Among the tree and shrub species, Prosopis juliflora showed the highest density (373.51 ind. /ha), frequency (63.50.67%), relative density (30.19.7%), relative frequency (24.41%) and relative abundance (7.68%).Regarding herb species, Aristida redacta represented the highest density (3.97ind./sq.m) and frequency (39.02%). Moreover, the highest importance value index was measured in Prosopis juliflora (62.28) among trees & shrubs and Aristida redacta (31.51) among herbs. The Abundance/Frequency ratio of trees, shrubs and herb species showed contagious distribution pattern within the study area. -

Flora-Lab-Manual.Pdf
LabLab MManualanual ttoo tthehe Jane Mygatt Juliana Medeiros Flora of New Mexico Lab Manual to the Flora of New Mexico Jane Mygatt Juliana Medeiros University of New Mexico Herbarium Museum of Southwestern Biology MSC03 2020 1 University of New Mexico Albuquerque, NM, USA 87131-0001 October 2009 Contents page Introduction VI Acknowledgments VI Seed Plant Phylogeny 1 Timeline for the Evolution of Seed Plants 2 Non-fl owering Seed Plants 3 Order Gnetales Ephedraceae 4 Order (ungrouped) The Conifers Cupressaceae 5 Pinaceae 8 Field Trips 13 Sandia Crest 14 Las Huertas Canyon 20 Sevilleta 24 West Mesa 30 Rio Grande Bosque 34 Flowering Seed Plants- The Monocots 40 Order Alistmatales Lemnaceae 41 Order Asparagales Iridaceae 42 Orchidaceae 43 Order Commelinales Commelinaceae 45 Order Liliales Liliaceae 46 Order Poales Cyperaceae 47 Juncaceae 49 Poaceae 50 Typhaceae 53 Flowering Seed Plants- The Eudicots 54 Order (ungrouped) Nymphaeaceae 55 Order Proteales Platanaceae 56 Order Ranunculales Berberidaceae 57 Papaveraceae 58 Ranunculaceae 59 III page Core Eudicots 61 Saxifragales Crassulaceae 62 Saxifragaceae 63 Rosids Order Zygophyllales Zygophyllaceae 64 Rosid I Order Cucurbitales Cucurbitaceae 65 Order Fabales Fabaceae 66 Order Fagales Betulaceae 69 Fagaceae 70 Juglandaceae 71 Order Malpighiales Euphorbiaceae 72 Linaceae 73 Salicaceae 74 Violaceae 75 Order Rosales Elaeagnaceae 76 Rosaceae 77 Ulmaceae 81 Rosid II Order Brassicales Brassicaceae 82 Capparaceae 84 Order Geraniales Geraniaceae 85 Order Malvales Malvaceae 86 Order Myrtales Onagraceae -

Scholar (Botany) University of Baluchistan Session:2017-2018
Mr.Hameedullah kakar M.sc: scholar (botany) university of Baluchistan session:2017-2018 FLOWER DESCRIPTION SOME IMPORTANT TERMS Parts of Flowers • The pistil has three parts: stigma, style, and ovary. • The stigma is the sticky surface at the top of the pistil; it traps and holds the pollen. • The style is the tube-like structure that holds up the stigma. • The style leads down to the ovary that contains the ovules. Classification of FLOWERS: • Complete: flowers possessing petals and sepals • Incomplete: flowers possessing either petals or sepals • Perfect: flowers containing both pistil and stamen • Imperfect: flowers containing either the pistil or stamen Parts of Flowers • A complete flower has a stamen, a pistil, petals, and sepals. • An incomplete flower is missing one of the four major parts of the flower, the stamen, pistil, petals, or sepals. Parts of Flowers • Flowers can have either all male parts, all female parts, or a combination. • Flowers with all male or all female parts are called imperfect (cucumbers, pumpkin and melons). • Flowers that have both male and female parts are called perfect (roses, lilies, dandelion). Students are to illustrate the following: • Complete/ Perfect Flower • Incomplete/Perfect Flower • Complete/ Imperfect Flower • Incomplete/ Imperfect Flower Types of Flowers: • As previously mentioned, there are plants which bear only male flowers (staminate plants) or bear only female flowers (pistillate plants). • Species in which the sexes are separated into staminate and pistillate plants are called dioecious. • Most holly trees and pistachio trees are dioecious; therefore, to obtain berries, it is necessary to have female and male trees. Types of Flowers: • Pistillate (female) flowers are those which possess a functional pistil(s) but lack stamens. -

Bentham and Hooker's Classification (1862 – ’83)
Bentham and Hooker's classification (1862 – ’83) George Bentham and Joseph Dalton Hooker - Two English taxonomists who were closely associated with the Royal Botanical Garden at Kew, England have given a detailed classification of plant kingdom, particularly the angiosperms. They gave an outstanding system of classification of phanerogams in their Genera Plantarum which was published in three volumes between the years 1862 to 1883. It is a natural system of classification. However, it does not show the evolutionary relationship between different groups of plants, in the strict sense. Nevertheless, it is the most popular system of classification particularly for angiosperms. The popularity comes from the face that very clear key characters have been listed for each of the families. These key characters enable the students of taxonomy to easily identify and assign any angiosperm plant to its family. Bentham and Hooker have grouped advanced, seed bearing plants into a major division called Phanerogamia. This division has been divided into three classes namely: 1. Dicotyledonae 2. Gymnospermae and 3. Monocotyledoneae 84 45 36 3 34 PhanerogamsSummaryor spermatophyta of Benthamare anddivided Hooker'sinto three classificationclasses - Dicotyledonae (1862 – 83), Gymnospermae and Monocotyledonae Class - Dicotyledonae– two cotyledons, open vascular bundles, reticulate venation I. Sub-Class Polypetalae - The flowers are usually with two distinct whorls of perianth; the segments of the inner whorl or "corolla" are free. A. Series-Thalamiflorae -(The calyx consists of usually distinct sepals, which are free from the ovary; doom shaped thalamus). 6 Orders/Cohort; 34 Families or Natural orders -R B. Series – Disciflorae - The calyx consists of either distinct or united sepals, which may be free or adnate to the ovary; a prominent ring of cushion shaped disc is usually present below the ovary, sometimes broken up into glands; the stamens are usually definite in number, inserted upon, or at the outer or inner base of the disc; the ovary is superior. -

Bibliographie Sur Les Genres Cistus L. Et Halimium (Dunal) Spach
1 Bibliographie sur les genres Cistus L. et Halimium (Dunal) Spach : Sommaire : Partie 1 : Liste de références bibliographiques classées par ordre alphabétique des auteurs ....p. 1- 136 Partie 2 : Liste de références bibliographiques avec le classement suivant : Généralités (Systématique, Nomenclature, Morphologie, Hybridation, Ladanum…), France, Régions-Départements, Europe, Iles Macaronésiennes, Afrique du Nord, Proche-Orient. ………………………………………...p. 136- 177 Partie 1 : 1. AAFI, A. 2007. Etude de la diversité floristique de l'écosystème de chêne-liège de la forêt de la Mamora. Thèse Doctorat es-Sciences Agronomiques. Institut Agronomique et Vétérinaire Hassan II, Rabat, Maroc.:190 p. 2. AAFI, A., A. ACHHAL EL KADMIRI, A. BENABID, and M. ROCHDI. 2005. Richesse et diversité floristique de la suberaie de la Mamora ( Maroc ). Acta Botanica Malacitana. 30:127-138. 3. ABBAS, H., M. BARBERO, R. LOISEL, and P. QUEZEL. 1985. Les forêts de pin d'Alep dans le Sud-Est méditerranéen français. Analyses écodendrologiques. Deuxième partie. Forêt Méditerranéenne. VII, n°2.:123-130. 4. ABBAYES, H. d. 1954. Le Chêne-vert (Quercus ilex L.) et son cortège floristique méditerranéen sur le littoral sud-ouest du Massif Armoricain. Vegetatio, La Haye. 5-6.:1-5. 5. ABBAYES, H. d., G. CLAUSTRES, R. CORILLION, and P. DUPONT. 1971. Flore et Végétation du Massif Armoricain. Saint-Brieuc : Presses Universitaires de Bretagne.: 258-260. 6. ABBAYES, H. d., and P. DUPONT. 1995. Supplément ( jusqu'à l'Année 1974 ) à la flore vasculaire du Massif Armoricain. E.R.I.C.A. 7 : 26. 7. ABI-SALEH, B., M. BARBERO, I. NAHAL, and P. QUEZEL. 1976. Les séries forestières de végétation au Liban. -

Bentham and Hooker's System of Angiosperm Classification
COMPILED AND CIRCULATED BY PROF. NANDITA BHAKAT, ASSISTANT PROFESSOR, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE BENTHAM AND HOOKER’S SYSTEM OF ANGIOSPERM CLASSIFICATION PROF. NANDITA BHAKAT Assistant professor Department of Botany Narajole Raj College BOTANY: SEM-IV, PAPER-C10T: PLANT SYSTEMATICS, UNIT-4: SYSTEMS OF CLASSIFICATION. COMPILED AND CIRCULATED BY PROF. NANDITA BHAKAT, ASSISTANT PROFESSOR, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE INTRODUCTION Classification denotes the arrangement of a single plant or group of plants an distinct category following a system of nomenclature, and in accordance with a particular and well established plan. Some of the earlier systems of classification of angiosperms were artificial systems, since they used only certain superficial characteristics as the basis. With more and more detailed study on the morphological, physiological and reproductive aspects of angiosperms, the artificial systems of classifications were replaced by the natural systems of classification. BOTANY: SEM-IV, PAPER-C10T: PLANT SYSTEMATICS, UNIT-4: SYSTEMS OF CLASSIFICATION. COMPILED AND CIRCULATED BY PROF. NANDITA BHAKAT, ASSISTANT PROFESSOR, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE George Bentham and Joseph Dalton Hooker - Two English taxonomists who were closely associated with the Royal Botanical Garden at Kew, England have given a detailed classification of plant kingdom, particularly the angiosperms. They gave an outstanding system of classification of phanerogams in their Genera Plantarum which was published in three volumes between the years 1862 to 1883. It is a natural system of classification. They described 97,205 species of flowering plants grouped into 202 orders (now recognised as families). The system has the advantage of being the first great natural system of classification, which is very easy to follow.