BIOL 154 Lab 4. Flower Formula and Flower Diagram
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Only Write Down the Correct Answer Next to the Appropriate Question Number on Your Answer Sheet
BOT1B10– November 2015 FACULTY OF SCIENCE DEPARTMENT OF BOTANY and PLANT BIOTECHNOLOGY MODULE PLANT DIVERSITY BOT1B10 CAMPUS APK EXAMINATION NOVEMBER 2015 DATE SESSION 14/November/2015 08:30 – 11:30 EXAMINER: PROF A. MOTEETEE INTERNAL MODERATOR: MRS J. WILLIAMSON DURATION: 3 HOURS MARKS: 120 ____________________________________________________________________________________ NUMBER OF PAGES: 11 PAGES INSTRUCTIONS: ANSWER ALL THE QUESTIONS _____________________________________________________________________________ QUESTION 1 [10] Choose an answer that matches the question the best: Only write down the correct answer next to the appropriate question number on your answer sheet. 1.1. Apart from food and beverages, plants provide human beings with: a) Oxygen, nitrogen, construction materials b) Medicines, essential oils, oxygen, fuel c) Paper, wool, cotton, silk d) Herb and spices, carbon, sodium, fodder for animals 1 BOT1B10– November 2015 1.2 Gymnosperms bear their seeds on the surfaces of: a) Leaves b) Cones c) Stems d) Fruits 1.3 Bryophytes have life cycles that depend on what for reproduction? a) Water b) Soil c) Grass d) Sun 1.4 Plants that have xylem and phloem are known as: a) Seed plants b) Photosynthetic plants c) Vascular-plants d) Non-vascular plants 1.5 Which on the following is the stalk by which the leaf blade is attached to the stem? a) Peduncle b) Pedicel c) Inflorescence d) Petiole 1.6 Seed plants use __________ and ___________ to reproduce. a) Pollen and seed b) Seeds and water c) Food and water d) Leaves and petals 1.7 -
1Lecture Notes 2013
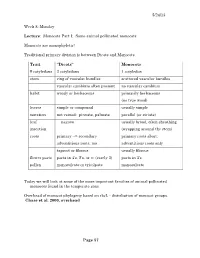
5/24/13 Week 8; Monday Lecture: Monocots Part I: Some animal pollinated monocots Monocots are monophyletic! Traditional primary division is between Dicots and Monocots Trait “Dicots” Monocots # cotyledons 2 cotyledons 1 cotyledon stem ring of vascular bundles scattered vascular bundles vascular cambium often present no vascular cambium habit woody or herbaceous primarily herbaceous (no true wood) leaves simple or compound usually simple venation net veined: pinnate, palmate parallel (or striate) leaf narrow usually broad, often sheathing insertion (wrapping around the stem) roots primary --> secondary primary roots abort; adventitious roots, too adventitious roots only taproot or fibrous usually fibrous flower parts parts in 4’s, 5’s, or ∞ (rarely 3) parts in 3’s pollen monosulcate or tricolpate monosulcate Today we will look at some of the more important families of animal pollinated monocots found in the temperate zone Overhead of monocot phylogeny based on rbcL - distribution of monocot groups. Chase et al. 2000, overhead Page 57 5/24/13 Lab only; limited discussion here. Show: “Plants are Cool, Too” video Araceae - Arum family (109 gen/2830 spp) 1) herbs (some epiphytes) 2) lvs simple or compound; broad and having an apparent petiole (‘pseudo-lamina’) development not same as in a dicot leaf blade 3) calcium oxalate crystals usually present – physical deterrent to herbivory 4) Inflorescence consisting of - spathe - bract (often colorful) surrounding the flowers - spadix - axis on which the flowers are borne (male above; female below, -

Downloaded on 12 March 2021, Was Applied to Evaluate the Extent of Species Other Than Chia in RNA-Seq Assemblies
plants Article Proteomic Identification and Meta-Analysis in Salvia hispanica RNA-Seq de novo Assemblies Ashwil Klein 1 , Lizex H. H. Husselmann 1 , Achmat Williams 1, Liam Bell 2 , Bret Cooper 3 , Brent Ragar 4 and David L. Tabb 1,5,6,* 1 Department of Biotechnology, University of the Western Cape, Bellville 7535, South Africa; [email protected] (A.K.); [email protected] (L.H.H.H.); [email protected] (A.W.) 2 Centre for Proteomic and Genomic Research, Cape Town 7925, South Africa; [email protected] 3 USDA Agricultural Research Service, Beltsville, MD 20705, USA; [email protected] 4 Departments of Internal Medicine and Pediatrics, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02150, USA; [email protected] 5 Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town 7500, South Africa 6 Centre for Bioinformatics and Computational Biology, Stellenbosch University, Stellenbosch 7602, South Africa * Correspondence: [email protected]; Tel.: +27-82-431-2839 Abstract: While proteomics has demonstrated its value for model organisms and for organisms with mature genome sequence annotations, proteomics has been of less value in nonmodel organisms that are unaccompanied by genome sequence annotations. This project sought to determine the value of RNA-Seq experiments as a basis for establishing a set of protein sequences to represent a nonmodel organism, in this case, the pseudocereal chia. Assembling four publicly available chia RNA-Seq datasets produced transcript sequence sets with a high BUSCO completeness, though the Citation: Klein, A.; Husselmann, number of transcript sequences and Trinity “genes” varied considerably among them. -

Print This Article
JOURNAL OF NATURAL REMEDIES DOI: 10.18311/jnr/2017/15945 Ethnobotanical Profiles and Phytochemical Constituents of Barringtonia racemosa L. for Potential Scrutiny of Bioactive Compounds through Plant Biotechnology Nurul Izzati Osman1, Norrizah Jaafar Sidik1* and Asmah Awal2 1Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia. 2Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia. Abstract This paper reviews the traditional uses and customs of people in the use of Barringtonia racemosa L., a type of plant mangrove species in their daily life and medicinal approaches. In addition, the phytochemical constituents and the studies in plant biotechnology carried out on this species were also reviewed and summarized by referring to the available documented literatures. It is aimed to provide a systematic review of such topics to facilitate understanding and providing information regarding that particular species. From the current review of literature, it has been clearly depicted that B. racemosa is having promising potentials for natural product discovery through plant biotechnology to be further scrutinized in this species. The information gathered from the ethnobotanical uses of this species as well as its phytochemical constituents are useful to provide a significant background for future works regarding plant secondary metabolites from this species to be further explored by the application of plant biotechnology. Keywords: Barringtonia racemosa L., Ethnobotany, Ethnopharmacology, Plant Bioactive Compounds, Phytochemistry 1. Introduction of plants not only lies in its medicinal properties used in herbal treatment but in fact, plants have been among the Our mother nature has a great diversity of plant species most significant element in daily household needs and and the existence of plant kingdom provides various being incorporated in industrial setting for instance in the benefits towards mankind. -

Arthur Monrad Johnson Colletion of Botanical Drawings
http://oac.cdlib.org/findaid/ark:/13030/kt7489r5rb No online items Arthur Monrad Johnson colletion of botanical drawings 1914-1941 Processed by Pat L. Walter. Louise M. Darling Biomedical Library History and Special Collections Division History and Special Collections Division UCLA 12-077 Center for Health Sciences Box 951798 Los Angeles, CA 90095-1798 Phone: 310/825-6940 Fax: 310/825-0465 Email: [email protected] URL: http://www.library.ucla.edu/libraries/biomed/his/ ©2008 The Regents of the University of California. All rights reserved. Arthur Monrad Johnson colletion 48 1 of botanical drawings 1914-1941 Descriptive Summary Title: Arthur Monrad Johnson colletion of botanical drawings, Date (inclusive): 1914-1941 Collection number: 48 Creator: Johnson, Arthur Monrad 1878-1943 Extent: 3 boxes (2.5 linear feet) Repository: University of California, Los Angeles. Library. Louise M. Darling Biomedical Library History and Special Collections Division Los Angeles, California 90095-1490 Abstract: Approximately 1000 botanical drawings, most in pen and black ink on paper, of the structural parts of angiosperms and some gymnosperms, by Arthur Monrad Johnson. Many of the illustrations have been published in the author's scientific publications, such as his "Taxonomy of the Flowering Plants" and articles on the genus Saxifraga. Dr. Johnson was both a respected botanist and an accomplished artist beyond his botanical subjects. Physical location: Collection stored off-site (Southern Regional Library Facility): Advance notice required for access. Language of Material: Collection materials in English Preferred Citation [Identification of item], Arthur Monrad Johnson colletion of botanical drawings (Manuscript collection 48). Louise M. Darling Biomedical Library History and Special Collections Division, University of California, Los Angeles. -

Toward a Phylogenetic-Based Generic Classification of Neotropical Lecythidaceae— I
Phytotaxa 203 (2): 085–121 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.203.2.1 Toward a phylogenetic-based Generic Classification of Neotropical Lecythidaceae— I. Status of Bertholletia, Corythophora, Eschweilera and Lecythis YA-YI HUANG1, SCOTT A. MORI2 & LAWRENCE M. KELLY3 1Institute of Plant and Microbial Biology, Academia Sinica, 128 Sec. 2, Academia Road, Taipei 11529, Taiwan; [email protected] 2Author for correspondence: Institute of Systematic Botany, The New York Botanical Garden, Bronx, New York, USA 10458-5126; [email protected] 3The New York Botanical Garden, Bronx, New York, USA 10458-5126; [email protected] Abstract Lecythidaceae subfam. Lecythidoideae is limited to the Neotropics and is the only naturally occurring subfamily of Le- cythidaceae in the New World. A subset of genera with zygomorphic flowers—Bertholletia, Corythophora, Eschweilera and Lecythis—comprises a group of about 125 species called the Bertholletia clade. A previous study based on plastid ndhF and trnL-F genes supported the monophyly of Corythophora but suggested that Eschweilera and Lecythis are not mono- phyletic. Using this study as a baseline, we sampled more taxa and sequenced more loci to address the taxonomic problems of the ambiguous genera and to determine relationships within the Bertholletia clade. Our results support the monophyly of the Bertholletia clade as previously circumscribed. In addition, Corythophora is monophyletic, and the two accessions of Bertholletia excelsa come out together on the tree. Results of the simultaneous analysis do not support the monophyly of Lecythis or Eschweilera. -

Tropical Plant-Animal Interactions: Linking Defaunation with Seed Predation, and Resource- Dependent Co-Occurrence
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2021 TROPICAL PLANT-ANIMAL INTERACTIONS: LINKING DEFAUNATION WITH SEED PREDATION, AND RESOURCE- DEPENDENT CO-OCCURRENCE Peter Jeffrey Williams Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Williams, Peter Jeffrey, "TROPICAL PLANT-ANIMAL INTERACTIONS: LINKING DEFAUNATION WITH SEED PREDATION, AND RESOURCE-DEPENDENT CO-OCCURRENCE" (2021). Graduate Student Theses, Dissertations, & Professional Papers. 11777. https://scholarworks.umt.edu/etd/11777 This Dissertation is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. TROPICAL PLANT-ANIMAL INTERACTIONS: LINKING DEFAUNATION WITH SEED PREDATION, AND RESOURCE-DEPENDENT CO-OCCURRENCE By PETER JEFFREY WILLIAMS B.S., University of Minnesota, Minneapolis, MN, 2014 Dissertation presented in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology – Ecology and Evolution The University of Montana Missoula, MT May 2021 Approved by: Scott Whittenburg, Graduate School Dean Jedediah F. Brodie, Chair Division of Biological Sciences Wildlife Biology Program John L. Maron Division of Biological Sciences Joshua J. Millspaugh Wildlife Biology Program Kim R. McConkey School of Environmental and Geographical Sciences University of Nottingham Malaysia Williams, Peter, Ph.D., Spring 2021 Biology Tropical plant-animal interactions: linking defaunation with seed predation, and resource- dependent co-occurrence Chairperson: Jedediah F. -

William Wayt Thomas1,2 & Melissa Tulig1
Rodriguésia 66(4): 983-987. 2015 http://rodriguesia.jbrj.gov.br DOI: 10.1590/2175-7860201566404 Hard Copy to Digital: Flora Neotropica and the World Flora Online William Wayt Thomas1,2 & Melissa Tulig1 Abstract One of the greatest challenges in achieving the goals of the World Flora Online (WFO) will be to make available the huge amount of botanical information that is not yet available digitally. The New York Botanical Garden is using the Flora Neotropica monograph series as a model for digitization. We describe our efforts at digitizing Flora Neotropica monographs and why digitization of hardcopy descriptions must be a priority for the WFO project. Key words: Electronic monographs, open access, Flora Neotropica, monographs. Resumo Um dos maiores desafios para alcançar as metas do projeto World Flora Online (WFO), será a disponibilizar a enorme quantidade de informações botânicas que ainda não estão disponíveis digitalmente. O New York Botanical Garden está utilizando a série de monografias da Flora Neotropica como um modelo para a digitalização. Nós aqui descrevemos nossos esforços na digitalização das monografias da Flora Neotropica e porque a digitalização das descrições impressas deve ser uma prioridade para o projeto WFO. Palavras-chave: Monografias eletrônicas, open access, Flora Neotropica, monografias. Introduction is called the World Flora Online (WFO). This consortium of professionals will create open- The World Flora Online (WFO) was access one-stop searching of world flora with developed as part of the United Nation’s Global verified information, including new and previously Strategy for Plant Conservation with the goal of published data, and coordinated with links to other providing “an online flora of all known plants,” One plant database and catalog Web sites. -

Secondary Successions After Shifting Cultivation in a Dense Tropical Forest of Southern Cameroon (Central Africa)
Secondary successions after shifting cultivation in a dense tropical forest of southern Cameroon (Central Africa) Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften vorgelegt beim Fachbereich 15 der Johann Wolfgang Goethe University in Frankfurt am Main von Barthélemy Tchiengué aus Penja (Cameroon) Frankfurt am Main 2012 (D30) vom Fachbereich 15 der Johann Wolfgang Goethe-Universität als Dissertation angenommen Dekan: Prof. Dr. Anna Starzinski-Powitz Gutachter: Prof. Dr. Katharina Neumann Prof. Dr. Rüdiger Wittig Datum der Disputation: 28. November 2012 Table of contents 1 INTRODUCTION ............................................................................................................ 1 2 STUDY AREA ................................................................................................................. 4 2.1. GEOGRAPHIC LOCATION AND ADMINISTRATIVE ORGANIZATION .................................................................................. 4 2.2. GEOLOGY AND RELIEF ........................................................................................................................................ 5 2.3. SOIL ............................................................................................................................................................... 5 2.4. HYDROLOGY .................................................................................................................................................... 6 2.5. CLIMATE ........................................................................................................................................................ -

11-FLOWER DIAGRAMES, FORMULAS and FLOWER SYMETRY FLOWER FORMULAS and DIAGRAMES
11-FLOWER DIAGRAMES, FORMULAS AND FLOWER SYMETRY FLOWER FORMULAS and DIAGRAMES 1. FLOWER FORMULAS Floral formula is a means to represent the structure of a flower using numbers, letters and various symbols, presenting substantial information about the flower in a compact form. It can represent particular species, or can be generalized to characterize higher taxa, usually giving ranges of organ numbers. Floral formulae are one of the two ways of describing flower structure developed during the 19th century, the other being floral diagrams. Apart from the graphical diagrams, the flower structure can be characterized by textual formulae. A floral formula consists of five symbols indicating from left to right: Floral Symmetry Number of Tepal Number of Sepals Number of Petals Number of Stamens Number of Carpels Tepals Sepals Patals Stamen Carpels P K C A G The parts of the flower are described according to their arrangement from the outside to the inside of the flower. If an organ type is arranged in more whorls, the outermost is denoted first, and the whorls are separated by “+”. If the organ number is large or fluctuating, is denoted as “∞”. 2. FLOWER DIAGRAMES Floral diagram is a graphic representation of flower structure. It shows the number of floral organs, their arrangement and fusion. Different parts of the flower are represented by their respective symbols. Rather like floral formulas, floral diagrams are used to show symmetry, numbers of parts, the relationships of the parts to one another, and degree of connation and/or adnation. Such diagrams cannot easily show ovary position. FLOWER SYMMETRY Floral symmetry describes whether, and how, a flower in particular its perianth, can be divided into two or more identical or mirror-image parts. -

Nested Whole-Genome Duplications Coincide with Diversification And
ARTICLE https://doi.org/10.1038/s41467-020-17605-7 OPEN Nested whole-genome duplications coincide with diversification and high morphological disparity in Brassicaceae Nora Walden 1,7, Dmitry A. German 1,5,7, Eva M. Wolf 1,7, Markus Kiefer 1, Philippe Rigault 1,2, Xiao-Chen Huang 1,6, Christiane Kiefer 1, Roswitha Schmickl3, Andreas Franzke 1, Barbara Neuffer4, ✉ Klaus Mummenhoff4 & Marcus A. Koch 1 1234567890():,; Angiosperms have become the dominant terrestrial plant group by diversifying for ~145 million years into a broad range of environments. During the course of evolution, numerous morphological innovations arose, often preceded by whole genome duplications (WGD). The mustard family (Brassicaceae), a successful angiosperm clade with ~4000 species, has been diversifying into many evolutionary lineages for more than 30 million years. Here we develop a species inventory, analyze morphological variation, and present a maternal, plastome-based genus-level phylogeny. We show that increased morphological disparity, despite an apparent absence of clade-specific morphological innovations, is found in tribes with WGDs or diversification rate shifts. Both are important processes in Brassicaceae, resulting in an overall high net diversification rate. Character states show frequent and independent gain and loss, and form varying combinations. Therefore, Brassicaceae pave the way to concepts of phy- logenetic genome-wide association studies to analyze the evolution of morphological form and function. 1 Centre for Organismal Studies, University of Heidelberg, Im Neuenheimer Feld 345, 69120 Heidelberg, Germany. 2 GYDLE, 1135 Grande Allée Ouest, Québec, QC G1S 1E7, Canada. 3 Department of Botany, Faculty of Science, Charles University, Benátská 2, 128 01, Prague, Czech Republic. -

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time.