Electronegativity Drawing Lewis Structures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Resonance, and Formal Charge
3.091: Introduction to Solid State Chemistry Maddie Sutula, Fall 2018 Recitation 8 1 Resonance Often, there are many ways that electrons can be arranged around a molecule. The existence of multiple electronic configurations that are stable leads to resonance, which describes the delocalization of electrons in covalently bonded molecules. To be considered resonance structures, molecules must meet the following criteria: 1. Atoms must be in the same orientation relative to each other 2. All resonance structures must have the same number of valence electrons 3. The octet rule must be satisfied, with a few exceptions: - Not followed by some elements in periods three and higher, including Cl, Br, I, P, Si − - Sulfur can have up to 12 e − - Boron can have 6 e 4. Formal charge should be on the most electronegative atoms 2− Example: Draw resonance structures for ozone (O3) and CO3 . Ozone consists of three oxygen atoms, for a total of 18 valence electrons. These can be equivalently dis- tributed in two ways: 2− CO3 consists of a central carbon atom which brings 4 valence electrons, and four oxygen atoms that each bring 6 electrons, and two additional electrons that yield the additional 2− charge. The resonance structures can be represented as follows: These equivalent structures can be combined into one compact picture (shown above on the right) that is an average of the possible structures. All of the resonance structures are equally likely, as they equivalently satisfy the guidelines for stable Lewis structures. 1 3.091: Introduction to Solid State Chemistry Maddie Sutula, Fall 2018 Recitation 8 2 Formal charge (again) 2− In the CO3 example above, the formal charge on each atom is shown in circles. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Constrained Density Functional Theory
Constrained Density Functional Theory The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Kaduk, Benjamin, Tim Kowalczyk, and Troy Van Voorhis. “Constrained Density Functional Theory.” Chemical Reviews 112.1 (2012): 321–370. © 2012 American Chemical Society As Published http://dx.doi.org/ 10.1021/cr200148b Publisher American Chemical Society (ACS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/74564 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Constrained Density Functional Theory Benjamin Kaduk, Tim Kowalczyk and Troy Van Voorhis Department of Chemistry, Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge MA 02139 May 2, 2011 Contents 1 Introduction 3 2 Theory 7 2.1 OriginalCDFTEquations ............................ 7 2.2 ConstrainedObservables . .. .. 9 2.3 ChoosingaConstraint .............................. 13 2.4 Implementation .................................. 18 2.5 Promolecules ................................... 24 2.6 Illustrations .................................... 28 2.6.1 MetalImpurities ............................. 28 2.6.2 Long-range Charge-Transfer Excited States . .... 32 2.7 FutureChallenges................................. 34 3 Application to Electron Transfer 36 3.1 Background:MarcusTheory. 36 3.2 DiabaticETStatesfromCDFT ......................... 39 3.2.1 Choosing Suitable Density Constraints for ET . 39 3.2.2 Illustrations ................................ 41 3.3 IncorporatingSolventEffects. .. 46 3.4 Molecular Dynamics and Free Energy Simulations . 49 3.5 RelatedandOngoingWork ........................... 54 4 Low-lying Spin States 57 4.1 TracingOutConstant-spinStates . .. 59 4.2 The Heisenberg Picture of Molecular Magnets . .. 63 4.3 Singlet-Triplet Gaps of Intermolecular CT States . -
Proceedings of the Indiana Academy of Science
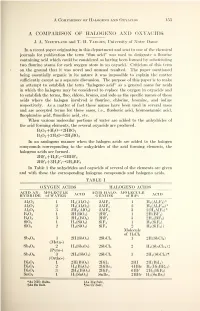
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Formal Charge Worksheet / Chem 314 Beauchamp
Formal Charge Worksheet / Chem 314 Beauchamp Formal Charge – a convention designed to indicate an excess or deficiency of electron density compared to an atom’s neutral allocation of electrons. Formal electrons in 1 electrons in Charge =Zeffective lone pairs 2 covalent bonds This number never changes for an atom and represents positive Total valence electrons allocated to an atom charge not cancelled assuming electrons are shared evenly in bonds. by core electrons. This number varies depending on the bonding arrangement. It is negative because electrons are negative. Rules of Formal Charge 1. When an atom’s total valence electron credit exactly matches its Zeff, there is no formal charge. 2. Each deficiency of an electron credit from an atom’s normal number of valence electrons produces an additional positive charge. 3. If the formal charge calculation shows excess electron credit over an atom’s normal number of valence electrons, a negative formal charge is added for each extra electron. 4. The total charge on an entire molecule, ion or free radical is the sum of all of the formal charges on the individual atoms. Write 3D structures for all resonance contributors. Rank them from best (=1) to last. Judge your structures on the basis of 1. maximum number of bonds / full octets, 2. minimize formal charge, 3, consistent formal charge based on electronegativity. Cations a. b. c. d. e. f. H COH 2 H2CNH2 (CH3)2COH (CH3)2CNHCH3 (CH3)2CCH2CH3 H2CCOH 2 resonance 2 resonance 2 resonance 2 resonance 2 resonance structures structures structures structures structures g. h. i. j. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Formal Charge and Oxidation Number
FORMAL CHARGE AND OXIDATION NUMBER Although the total number of valence electrons in a molecule is easily calculated, there is not aways a simple and unambiguous way of determining how many reside in a particular bond or as non-bonding pairs on a particular atom. For example, one can write valid Lewis octet structures for carbon monoxide showing either a double or triple bond between the two atoms, depending on how many nonbonding pairs are placed on each: C::O::: and :C:::O: (see Problem Example 3 below). The choice between structures such as these is usually easy to make on the principle that the more electronegative atom tends to surround itself with the greater number of electrons. In cases where the distinction between competing structures is not all that clear, an arbitrarily-calculated quantity known as the formal charge can often serve as a guide. The formal charge on an atom is the electric charge it would have if all bonding electrons were shared equally with its bonded neighbors. How to calculate the formal charge on an atom in a molecule The formal charge on an atom is calculated by the following formula: In which the core charge is the electric charge the atom would have if all its valence electrons were removed. In simple cases, the formal charge can be worked out visually directly from the Lewis structure, as is illustrated farther on. Problem Example 1 Find the formal charges of all the atoms in the sulfuric acid structure shown here. Solution: The atoms here are hydrogen, sulfur, and double- and single-bonded oxygens. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

Chemical Bonding
Chemical Bonding: Fundamental Concepts Resonance Structures and Formal Charge Electronegativity, Formal Charge and Resonance Page [1 of 3] In this lecture we’re going to pull together ideas about formal charge, resonance structures, electronegativity, and really make some predictions and some rationalizations about why molecules behave the way they do. And the first one I want to do is to go back and look at the cyanate ion. Cyanate is NCO. And in a previous lecture I talked about the fact that there were several different possible resonance structures that are all in equivalence, and that it was possible at least to figure out which one contributed the most and which one contributed the least based on formal charge. So here are the formal charge evaluations for this left-hand resonance structure. Nitrogen has a formal charge of zero. Carbon has a formal charge of zero. Oxygen has a formal charge of -1. For B, nitrogen is -1, carbon is zero, oxygen is zero. And for C, nitrogen is -2, carbon is zero, and oxygen is +1. Now, the rule said that formal charges of plus or minus 1 and zero are okay. In fact, zero is great. And plus or minus 2 and bigger, that’s just not going to work. Why? Because remember, formal charges reflect how the electrons are distributed relative to how they are distributed in the free atom. So how they’re distributed in a molecule versus how they’re distributed in the free atom. And in this case nitrogen has a lot more electrons than it would if were a free atom formally; in other words, an accounting method. -
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
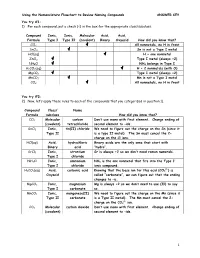
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes. -
Curricular Axis Standard Competencies
SUBJECT GRADE LEARNING UNIT NATURAL SCIENCES 9TH HOW TO RELATE THE COMPONENTS OF THE WORLD TITLE OF LEARNING OBJECT How does water transform the minerals in rocks CURRICULAR AXIS Living environment STANDARD Record my observations and results by using diagrams, graphs COMPETENCIES and tables. LEARNING OBJECTIVES 1. To make short presentations of the acid compounds (oxyacid and hydracids) and hydroxides contained in products used in the industry or at home. 2. To explain in short conversations, the influence of acids (oxyacids- hydracids) and hydroxides in natural and human- induced phenomena in the planet. 3. To establish relations between metals, non-metals and the formation of acids and hydroxides. SKILLS/ KNOWLEDGE S/K 1: Illustrate the paths to obtain acids (oxyacids and hydracids) and hydroxides and establishes relations with the organization of the periodic table of elements. S/K2: Provide examples of applications and uses of acids and hydroxides. S/K3: Differentiate the various ways in which an acid or a hydroxide can be named. S/K4: Represent and describes the chemical equations of salt formation. S/K 5: Explain acid rain and inquires about the natural and human processes behind it. S/K 6: Enquire about the weathering process. LEARNING FLOW Introduction Objectives Activity 1: Obtaining acids ( oxyacids - hydracid) and hydroxides Salt formation Applications in industry and domestic activities Activity 2: Nomenclature of acids (oxyacids - hydracid), hydroxides and salt. Activity 3: •Acid rain • Weathering Abstract Homework Evaluation Glossary ASSESSMENT This learning object is intended for the student to find a simple, GUIDELINE didactic and contextual guide. Likewise, students must develop the guide completely, including the different activities of this LO, by using the diverse tools and autonomous work suggested here.