Low Cost Photo Bioreactor for Marine Microalga Cutivation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why I Became a Hindu
Why I became a Hindu Parama Karuna Devi published by Jagannatha Vallabha Vedic Research Center Copyright © 2018 Parama Karuna Devi All rights reserved Title ID: 8916295 ISBN-13: 978-1724611147 ISBN-10: 1724611143 published by: Jagannatha Vallabha Vedic Research Center Website: www.jagannathavallabha.com Anyone wishing to submit questions, observations, objections or further information, useful in improving the contents of this book, is welcome to contact the author: E-mail: [email protected] phone: +91 (India) 94373 00906 Please note: direct contact data such as email and phone numbers may change due to events of force majeure, so please keep an eye on the updated information on the website. Table of contents Preface 7 My work 9 My experience 12 Why Hinduism is better 18 Fundamental teachings of Hinduism 21 A definition of Hinduism 29 The problem of castes 31 The importance of Bhakti 34 The need for a Guru 39 Can someone become a Hindu? 43 Historical examples 45 Hinduism in the world 52 Conversions in modern times 56 Individuals who embraced Hindu beliefs 61 Hindu revival 68 Dayananda Saraswati and Arya Samaj 73 Shraddhananda Swami 75 Sarla Bedi 75 Pandurang Shastri Athavale 75 Chattampi Swamikal 76 Narayana Guru 77 Navajyothi Sree Karunakara Guru 78 Swami Bhoomananda Tirtha 79 Ramakrishna Paramahamsa 79 Sarada Devi 80 Golap Ma 81 Rama Tirtha Swami 81 Niranjanananda Swami 81 Vireshwarananda Swami 82 Rudrananda Swami 82 Swahananda Swami 82 Narayanananda Swami 83 Vivekananda Swami and Ramakrishna Math 83 Sister Nivedita -

6433-6440 E-ISSN:2581-6063 (Online), ISSN:0972-5210
Plant Archives Volume 20 No. 2, 2020 pp. 6433-6440 e-ISSN:2581-6063 (online), ISSN:0972-5210 ETHNO-BOTANICAL SIGNIFICANCE OF HINDU HOLY PLANTS IN KANYAKUMARI DISTRICT, TAMILNADU, INDIA S. Mary Sheeja and J. Lohidas* Scott Christian College (Autonomous) Nagercoil- 629003, Affiliated toManonmaniam Sundaranar University, Abishekapatti, Tirunelveli-627012 (Tamil Nadu), India Associated Professor, (Department of Botany) Scott Christian College (Autonomous) Nagercoil- 629003, Affiliated to Manonmaniam Sundaranar University, Abishekapatti, Tirunelveli-627012 (Tamil Nadu), India. Abstract Ethno-botanical studies carried out in Traditional and medicinal practices of the people in Western Ghats. During this study there are 44 plants belongs to 29 families and 42 genera were identified as medicinal plants were used to treat Blood sugar, Snake bites, Antibacterial, anti microbial, anti viral, Paralysis, Gastrointestinal disorders, Malaria, Head ache, regularize menstrual cycle, Asthma, Chronic ulcers and Antiseptic. The plant parts were used as decoction, powder is mainly used form of medicine in the study area. Plants part of leaves, stem, bark, seed oil, root, seed, flower and rhizome are also agreed by the ethno-botanical researches. Key words: Ethno-botany, Medicinal plants, Western Ghats. all over the world have been recognized for their ethno- Introduction medicinal importance in India about 2000 plants are used Ethno-botanical studies range across space and time medicinal purpose for medicinal healers. The traditional from archeological investigations of the role of plants in knowledge on the herbal drug has been orally transmitted ancient civilizations. It comprises both wild and from one generation to a different generation. domesticated species and is rooted in observation, In Bhagavad Gita, the Hindu holy book, instructs relationship, needs and traditional ways of such knowledge some flowers correspond to specific gods and should only evolves over time, and is therefore always varying and be used for certain days or rituals. -

Religions Manual Development of New Inter-Religious Tools
This Publication is part of the project Development of new Inter-religious tools. HOLY MEMO Development of new Inter-religious tools Religions Manual Development of new Inter-religious tools HOLY MEMO This Manual is part of the game HOLY MEMO which is created during project Development of new Inter-religious tools Development of new Inter-religious tools is a project financed under Key Action 2, Capacity building in the field of youth under Western Balkans Window by European Commission, within Erasmus + Youth in Action Program. Project partners: NGO Iuventa (Serbia); Associazione TDM 2000 (Italy); Beyond Barriers - Pertej Barrierave (Albania); BEES (Austria); Intercultura Dinan (France); United Societies of Balkans (Greece); Batman Fen Lisesi Mezunları Derneği (Turkey); Föreningen Framtidståget (Sweden); Better Life In Kosova (Kosovo); Crveni Križ NOVO SARAJEVO (Bosnia and Herzegovina); Nvo Prima (Montenegro) "The European Commission support for the production of this publication does not constitute an endorsement of the contents which reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein." 1 2 TABLE OF CONTENTS AYYAVAZHI ............................................................................................................................................. 4 BAHA'I FAITH .......................................................................................................................................... 5 BUDDHISM ............................................................................................................................................... -

Andhra Pradesh
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE BALAJI COMPLEX OPP. RAGHAVA THEATRE JAILKHANA CHITTOOR ROAD CHITTOOR – CIR@FED ANDHRA (ANDHRA 517 001 ANDHRA ERALBANK PRADESH CHITTOOR PRADESH) PRADESH CHITTOOR FDRL0001673 .CO.IN D.NO.6-7-587 0877 - A.K.NILAYAM 2231361 SRIPURAM COLONY TRP@FED ANDHRA ISKCON ROAD ERALBANK PRADESH CHITTOOR TIRUPATI TIRUPATI - 517 501 TIRUPATI FDRL0001683 .CO.IN AYYAPPA TOWERS (PHASE II), D. NO. 5 - 1 - 76, MAIN ROAD, OPP. TWO TOWN POLICE STATION, KAKINADA - 533001, EAST GODAVARI DURGA ANDHRA EAST DISTRICT, ANDHRA PRASADA KNA@FEDERA 0884 PRADESH GODAVARI KAKINADA PRADESH KAKINADA FDRL0001626 RAO K LBANK.CO.IN 2363611 SWAROOP GEORGE P, E DOOR NO: 60506, MAIL:RMD MAIN ROAD, @FEDERA OPP.SHYAMALA LBANK.CO. TALKIES, IN PH NO.: RAJAMUNDRY, 533 0883- ANDHRA EAST 101, ANDHRA RAJAHMUN 2461457(M PRADESH GODAVARI RAJAHMUNDRY PRADESH DRY FDRL0001341 ) 0863 – 2332277, 2332278 6-14-36, 14TH LANE GTR@FED ANDHRA ARUNDELPET ERALBANK PRADESH GUNTUR GUNTUR GUNTUR – 522 002 GUNTUR FDRL0001671 .CO.IN GOPAKUM AR P, E MAIL:HYD @FEDERA P B NO. 230, ORIENT LBANK.CO. ESTATE, ABIDS IN PH NO.: ROAD, HYDERABAD, 040- ANDHRA 500 001, ANDHRA HYDERABA 23205381( PRADESH HYDERABAD HYDERABAD PRADESH D FDRL0001124 CM) GROUND FLOOR, D.NO. 16-11- 20/6/6/1/1, OPP. TV TOWER, GAFOOR BAGH, SALEEM NAGAR COLONY, MALAKPET, HYDERABAD - HYDG@FE ANDHRA HYDERABAD / 500036, ANDHRA HYDERABA 040 DERALBA PRADESH HYDERABAD MALAKPET PRADESH D FDRL0001826 24540022 040 24540000 NK.CO.IN NO 301 III FLOOR 040 DEGA TOWERS 23303305 RAJBHAVAN -
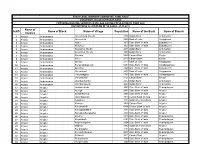
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

Download Download
Article CASTE: A Global Journal on Social Exclusion Vol. 1, No. 1, pp. 125–154 February 2020 brandeis.edu/j-caste ISSN 2639-4928 DOI: 10.26812/caste.v1i1.96 Mirrors of the Soul Performative Egalitarianisms and Genealogies of the Human in Colonial-era Travancore, 1854-1927 Vivek V. Narayan1 (Bluestone Rising Scholar 2019 Award) for Pradeepan Pampirikunnu, with gratitude and in loving memory of an all-too-brief conversation Abstract Scenes of avarna castes (slave and intermediate castes) pondering their reflections recur throughout the history of anti-caste struggle in the princely state of Travancore in colonial-era south India. These scenes represent what I will call performative egalitarianisms, which are repetitive enactments in the performance of everyday lives that embody claims to equality against the dehumanizing caste codes of colonial Travancore. In this paper, I will describe three scenes that represent distinct yet intertwined routes for the flows of egalitarian discourses in colonial Kerala. The concept of equality emerged in Travancore, first, via Enlightenment values of the British Protestant missionaries, or soulful Enlightenment; second, as non-dualistic equality of Narayana Guru, or repurposed Advaita; and third, through the discourses and practices of a Tamil religious cult called Ayya Vazhi, or radical Siddha Saiva. In viewing the flows of egalitarian discourse through the lens of performance, I demonstrate the method of intellectual histories in the repertoire which allows us to investigate how particular conceptual frameworks and discursive modes are transmitted, transformed, and embodied by people for whom these ideas are, quite literally, a matter of life and death. The intentional, productive, and empowering relationship between universals such as equality or humanity and the particular claims of anti-caste struggle in Kerala leads to a politics of practice that I describe as repurposing universals. -

Temples Within Chennai City
Temples within Chennai City 1 As the famous Tamil poetess AUVAYYAR says in Her Legendary presentation of cluster of hymns “Kovil illatha ooril kudi irukkathe” Please don’t reside in a place where there is no temple. The Statement of our forefathers is sacrosanct because the temple indicates that the community is graced by the presence of God and that its Citizens form a moral community. A Community identifies and is identified by others with its temples. It has been our ancient endavour to lead a pious life with full dedication to the services of the Lord. Sri Paramacharya of Kanchi Mutt has repeatedly called devotees and stressed the importance of taking care of old temples - which requires enormous power of men and money - instead of constructing new temples in cities. As you may be aware, there are thousands of temples in dilapidated condition and requires constant maintenance work to be undertaken. There are many shiva lingas of ancient temples found under trees and also while digging. In ancient times, these lingas were 'Moolavars' of temples built by several kings. After conquests and devastations by foreign invaders, Indian temples were destructed and the sacred deities were thrown away and many were broken. The left out deities are found later. Of them, some are unidentified. Those who attempt to construct temples for gods are freed from the sins of a thousand births. Those who think of building a temple in their minds are freed from the sins of a hundred births. Those who contribute to the cause of a temple are bestowed with divine virtues and blessings. -

Kanniyakumari
Census of India 2011 TAMIL NADU PART XII-A SERIES-34 DISTRICT CENSUS HANDBOOK KANNIYAKUMARI VILLAGE AND TOWN DIRECTORY DIRECTORATE OF CENSUS OPERATIONS TAMIL NADU CENSUS OF INDIA 2011 TAMIL NADU SERIES 34 PART XII-A DISTRICT CENSUS HANDBOOK KANNIYAKUMARI VILLAGE AND TOWN DIRECTORY Directorate of Census Operations Tamil Nadu 2011 VIVEKANANDA MEMORIAL AND THIRUVALLUVAR STATUE There are two rocks projecting out of the Indian Ocean, south- east of Kanniyakumari temple. These rocks provide an ideal vantage point for visitors desiring to view the land end of India. On one of these rocks, Swami Vivekananda sat in long and deep meditation, when he visited Kanniyakumari in 1892. On this rock, the “Vivekananda Rock Memorial” was built in 1970 with a blend of all the architectural styles of India. A statue of Swami Vivekananda has been installed inside this memorial building. One can also see “Sri Padha Parai”, believed by the devout to be the foot prints of the virgin Goddess Kanniyakumari on this rock. The Thiruvalluvar Statue is a 133 feet tall stone sculpture of the Tamil poet and philosopher, Tiruvalluvar, author of the Thirukkural located adjacent to Vivekananda Rock Memorial. DISTRICT CENSUS HANDBOOK - 2011 CONTENTS Page Foreword i Preface iii Acknowledgements iv History and Scope of the District Census Handbook v Brief History of the District vi Highlights of the District - 2011 Census vii Important Statistics of the District - 2011 Census viii Analytical Note 1 Village and Town Directory 103 Brief Note on Village and Town Directory 105 Section -I Village Directory 111 (a) List of villages merged in towns and outgrowths at 2011 Census 112 (b) C.D. -

INDIAN OVERSEAS BANK Villages Having Population Over 2000 Identified and Allotted to Banks for Extension of Banking Services
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK Villages having Population over 2000 identified and allotted to Banks for extension of Banking Services Name of S.NO. Name of Block Name of Village Population Name of Bank Name of Branch District 1 Ariyalur Sendurai Adhanakurichi 3528 State Bank of India Asaveerankudikadu 2 Ariyalur Tirumanur Alagiyamanavalam 3570 Bank of India Elakurichi 3 Ariyalur Sendurai Alathiyur 4012 State Bank of India Asaveerankudikadu 4 Ariyalur T.Palur Ammbappur 3029 Indian Bank Udayarpalayam 5 Ariyalur T.Palur Anaikudam 4182 State Bank of India Udayanatham 6 Ariyalur Sendurai Anandavadi 3854 State Bank of India Sendurai 7 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 8 Ariyalur Jayamkondacholapuram Angarayanallur (East) 2944 Canara Bank JKC 9 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 10 Ariyalur Tirumanur Annimangalam 2868 Canara Bank Thirumazhapadi 11 Ariyalur Ariyalur Ariyalur (North) 3221 State Bank of India Ariyalur 12 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 13 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 14 Ariyalur Sendurai Ayanathathanur 3411 State Bank of India Asaveerankudikadu 15 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 16 Ariyalur Tirumanur Chinnapatakadu 2417 Bank of India Elakurichi 17 Ariyalur T.Palur Cholamadevi 3458 Canara Bank Kodalikarupur 18 Ariyalur T.Palur Devamangalam 3137 State Bank of India Udayanatham 19 Ariyalur Andiamadam Edaayankurichi 4015 State Bank -

District Census Handbook, Kanniyakumari, Part XIII a and B
CENSUS OF INDIA 1981 SERIES 20 TAMIL NADU PART XIII A and B DISTRICT CENSUS HANDBOOK VILLAGE AND TOWN DIRECTORY AND VILLAGE AND TOWNWISE PRIMARY CENSUS ABSTRACT KANNIY AKUMARI A. P. lVIUTHUSWAMI of the Indian Administrative Service Director· of Census Operations Tamil Nadu . Price: PUBL1SHED BY GOVERNMENT OF l'A,MH. NADU AND' PRINTED BY TaE DIRECTOR. OF STATIONERY AND PRINTIN<1 AT GOVeRNMENT JlRESS, MADRAS·600 G79 GONTENTS PAGES 1. Foreword iii-iv 2. Preface v-vi 3. Acknowledgement vii 4. District Map Facing P. ix S. Important Statistics ix-x 6. Analytical Note- (i) Census Concepts :-Rural and Urban Areas. Census house I household Scheduled Castes Scheduled Tribes, Literate. Main Worker, Marginal Worker, Non worker etc. 1-2 (ii) History o~ the District C~nsus Handbook including scope of Village and Town DIrectory and Pnmary Census Abstract . .. 2-6 (iii) Early history, Physic".! features, location and physiography soils, climate and rainf211, Hills, Rivers, Coastline, Geology and Minerals, Forestry, Land and Land ust: pattern, Agriculture, Irrigation and power. Animal Husbandry, Fishery, IndustrY-Medium and large industry, Cottage Industry, Trade, Transport and Communication, Social and cultural event, places of tourist interest .. 7-15 7 Brief analy~is of the Village, Town Directory and Primary Census Abstract data. 16-26 pART A-VILLAGE AND TOWN DIRECTOll.Y SEOTION I VILLAGE DIRECTORY Note explaining th.:: codes used in the village Direotory 29-30 1. Vilavankod Taluk (i) Taluk map .. •• Facing P. 32 (ii) Alphabetical list of Village 33 (iii) Village Directory Statement 34-37 2. Kalkulam Taluk (i) Taluk Map .. •• Facing P. -

List of Atms Enabled with Ramp Facility.Xlsx
LIST OF FEDERAL BANK ATMS ENABLED WITH RAMP FACILITY SL No: ATM -ID Location State 1 FD140201 NSS BUILDING, PERUNNA Kerala 2 CD155201 FEDERAL BANK LTD, MADHUBAN,UDAIPUR Rajasthan 3 FD182101 52 DR. B N SAIKIA ROAD, SURVEY, BELTOLA Assam 4 FD201401 SHIVARAM COMPLEX, NEAR LD BUS STAND, MUNICIPAL MAIN ROAD, KUNDAPURA Karnataka 5 FD139805 ST.JOHN BAPTIST ROAD, BANDRA, MUMBAI Maharashtra 6 CD216601 THE FEDERAL BANK LTD, BILLAPURA BRANCH, SY NO 20, THYVAKANAHALLI, BILLAPURA Karnataka 7 CD103916 CITADEL RESIDENTIAL SCHOOL,ITTICHUVADU(PO),RANNY Kerala 8 CD109501 P B NO. 22 MUTTOM BAZAR CHERTHALA ALAPUZHA, KERALA Kerala 9 CD156301 40 , TRUNK ROAD ,POONAMALLEE , CHENNAI Tamilnadu 10 CD124401 SREEDURGA X/598A,RAILWAY STATION ROAD,NELLAYI,IRINJALAKUDA Kerala 11 CD151601 KERALA SAMAJAM MODEL SCHOOL CAMPUS, SAKCHI , JAMSHEDPUR Jharkhand 12 CD132401 C48,T.N.H.B COMPLEX ,SECOND AVENUE,ANNA NAGAR Tamilnadu 13 CD216801 NO. 168/169, B NANJAPPA LAYOUT, SURYA NAGAR, BANGALORE Karnataka 14 FD204801 KINGINI BUILDINGS, OPP. ST THOMAS FORANE CHURCH,PUTHUR Kerala 15 CD226201 NO 84, SAMETHANE POST, BANGALORE Karnataka 16 CD153201 FATHIMA COMPLEX,PATTAMBI ROAD, CHERPULASSERY Kerala 17 CD125101 ASHA BUILDING, NEAR GOVT. HIGH SCHOOL, MANNANCHERRY, ALAPPUZHA Kerala 18 CD155501 PLOT NO.80 & 81, ARUNODAYA COLONY. telengana 19 CD214301 GOPAL PLAZA, 11, VARRUCHI MARG, NEAR SHAHEED PARK, FREEGANJ madhya pradesh 20 CD222201 ITARSI BRANCH, 11TH LINE, ITARSI HOSHANGABAD madhya pradesh 21 CD158701 BHAGWANI COMPLEX, NAJAHGARH ROAD, MAIN BAZAR, BAHADURGARH , JHAJJAR DIST haryana -

District Census Handbook, Kanniyakumari, Part XII-A & B
CENSUS OF INDIA 2001 SERIES-33 TAMIL NADU DISTRICT CENSUS HANDBOOK Part -A & B KANNIYAKUMARI DISTRICT VILLAGE & TOWN DIRECTORY ~ VILLAGE AND TOWNWISE PRIMARY CENSUS ABSTRACT Dr. C. Chandramouli of the Indian Administrative Service Director of Census Operations, Tamil Nadu VIVEKANANDA MEMORIAL There are two rocks projecting out of the Indian Ocean, south-east of Kanniyakumari temple. These rocks provide an ideal vantage point for visitors desiring to view the land end of India. On one of these rocks, Swami Vivekananda sat in long and deep meditation, when he visited Kanniyakumari in 1892. On this rock, the "Vivekananda Rock Memorial" was built in 1970 with a blend of all the architectural styles of India. A statue of Swami Vivekananda has been installed inside this memorial building. One can also see "Sri Padha Parai", believed by the devout to be the foot prints of the virgin Goddess Kanniyakumari on this rock. (iii) Contents Pages Foreword ix Preface xi Acknmvledgements xm Map ofKanniyakumari District xv District Highlights - 2001 XVll Important Statistics of the District Xl)( Ranking ofTaluks in the District XXI Summary Statements 1 - 9 Statement 1 Name of the headquarters of DistrictlTaluk, their rural-urban xxiv status and distance from District headquarters, 2001 Statement 2 Name of the headquarters of District/CD block, their XXIV rural-urban status and distance from District headquarters, 2001 Statement 3 Population of the District at each census from 1901 to 2001 xxv Statement 4 Area, number of villages/towns and population in