UNIVERSITY of CALIFORNIA, SAN DIEGO Biochemical and Structural
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CISD2 (NM 001008388) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC207131L3 CISD2 (NM_001008388) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: CISD2 (NM_001008388) Human Tagged ORF Clone Tag: Myc-DDK Symbol: CISD2 Synonyms: ERIS; Miner1; NAF-1; WFS2; ZCD2 Vector: pLenti-C-Myc-DDK-P2A-Puro (PS100092) E. coli Selection: Chloramphenicol (34 ug/mL) Cell Selection: Puromycin ORF Nucleotide The ORF insert of this clone is exactly the same as(RC207131). Sequence: Restriction Sites: SgfI-MluI Cloning Scheme: ACCN: NM_001008388 ORF Size: 405 bp This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 CISD2 (NM_001008388) Human Tagged ORF Clone – RC207131L3 OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_001008388.1 RefSeq Size: 5892 bp RefSeq ORF: 408 bp Locus ID: 493856 UniProt ID: Q8N5K1 Protein Families: Transmembrane MW: 15.3 kDa Gene Summary: The protein encoded by this gene is a zinc finger protein that localizes to the endoplasmic reticulum. -
Primepcr™Assay Validation Report
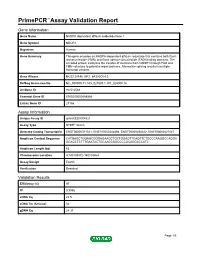
PrimePCR™Assay Validation Report Gene Information Gene Name NADPH dependent diflavin oxidoreductase 1 Gene Symbol NDOR1 Organism Human Gene Summary This gene encodes an NADPH-dependent diflavin reductase that contains both flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) binding domains. The encoded protein catalyzes the transfer of electrons from NADPH through FAD and FMN cofactors to potential redox partners. Alternative splicing results in multiple transcript variants. Gene Aliases MGC138148, NR1, bA350O14.9 RefSeq Accession No. NC_000009.11, NG_027801.1, NT_024000.16 UniGene ID Hs.512564 Ensembl Gene ID ENSG00000188566 Entrez Gene ID 27158 Assay Information Unique Assay ID qHsaCED0004821 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000371521, ENST00000344894, ENST00000458322, ENST00000427047 Amplicon Context Sequence CATGAGCTGGAGCGGGAGAAGCTGCTGGAGTTCAGTTCTGCCCAAGGCCAGGA GGAGCTCTTTGAATACTGCAACCGGCCCCGCAGGACCATC Amplicon Length (bp) 63 Chromosome Location 9:140109372-140109464 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 97 R2 0.9996 cDNA Cq 22.5 cDNA Tm (Celsius) 82 gDNA Cq 24.37 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report NDOR1, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 3/5 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Cytotoxic Effects and Changes in Gene Expression Profile
Toxicology in Vitro 34 (2016) 309–320 Contents lists available at ScienceDirect Toxicology in Vitro journal homepage: www.elsevier.com/locate/toxinvit Fusarium mycotoxin enniatin B: Cytotoxic effects and changes in gene expression profile Martina Jonsson a,⁎,MarikaJestoib, Minna Anthoni a, Annikki Welling a, Iida Loivamaa a, Ville Hallikainen c, Matti Kankainen d, Erik Lysøe e, Pertti Koivisto a, Kimmo Peltonen a,f a Chemistry and Toxicology Research Unit, Finnish Food Safety Authority (Evira), Mustialankatu 3, FI-00790 Helsinki, Finland b Product Safety Unit, Finnish Food Safety Authority (Evira), Mustialankatu 3, FI-00790 Helsinki, c The Finnish Forest Research Institute, Rovaniemi Unit, P.O. Box 16, FI-96301 Rovaniemi, Finland d Institute for Molecular Medicine Finland (FIMM), University of Helsinki, P.O. Box 20, FI-00014, Finland e Plant Health and Biotechnology, Norwegian Institute of Bioeconomy, Høyskoleveien 7, NO -1430 Ås, Norway f Finnish Safety and Chemicals Agency (Tukes), Opastinsilta 12 B, FI-00521 Helsinki, Finland article info abstract Article history: The mycotoxin enniatin B, a cyclic hexadepsipeptide produced by the plant pathogen Fusarium,isprevalentin Received 3 December 2015 grains and grain-based products in different geographical areas. Although enniatins have not been associated Received in revised form 5 April 2016 with toxic outbreaks, they have caused toxicity in vitro in several cell lines. In this study, the cytotoxic effects Accepted 28 April 2016 of enniatin B were assessed in relation to cellular energy metabolism, cell proliferation, and the induction of ap- Available online 6 May 2016 optosis in Balb 3T3 and HepG2 cells. The mechanism of toxicity was examined by means of whole genome ex- fi Keywords: pression pro ling of exposed rat primary hepatocytes. -

Assessment of Genetic Mutations in WFS1 & CISD2 in Wolfram
Online Journal of Neurology and L UPINE PUBLISHERS Brain Disorders Open Access DOI: 10.32474/OJNBD.2018.01.000104 ISSN: 2637-6628 Research Article Assessment of Genetic Mutations in WFS1 & CISD2 in Wolfram Syndrome Human Shahin Asadi*, Mahsa Jamali, Hamideh Mohammadzadeh and Mahya Fattahi Director of the Division of Medical Genetics and Molecular Research, Iran Received: January 25, 2018; Published: February 15, 2018 *Corresponding author: Shahin Asadi, Molecular Genetics Iran Tabriz, Director of the Division of Medical Genetics and Molecular Research, Iran Abstract In this study we have analyzed 30 people. 10 patients Wolfram syndrome and 20 persons control group. The genes WFS1 and CISD2, analyzed in terms of genetic mutations made. In this study, people who have genetic mutations were targeted, with nervous disorders Wolfram syndrome. In fact, of all people with Wolfram syndrome. 10 patients Wolfram syndrome had a genetic mutation in the genes WFS1 and CISD2 Wolfram syndrome. Any genetic mutations in the target genes control group did not show. Keywords: Genetic study; Wolfram syndrome; Mutations The gene WFS1 and CISD2; RT-PCR. Generalizations of Wolfram Syndrome urinary tract, decreased testosterone levels in men (hypogonadism), Wolfram syndrome is a genetic disorder that affects many body Nervous or psychiatric disorders [1]. systems. The specific characteristics of Wolfram syndrome include: mellitus) and progressive vision loss due to degeneration of the syndrome, which is commonly diagnosed at about 6 years of age. high blood sugar levels due to insulin hormone deficiency (diabetes Diabetes mellitus is usually the first symptom of Wolfram nerves that transports visual information from the eye to the brain Almost all people with Wolfram syndrome who have diabetes need (vision atrophy). -

Wolfram Syndrome
Wolfram syndrome Description Wolfram syndrome is a condition that affects many of the body's systems. The hallmark features of Wolfram syndrome are high blood sugar levels resulting from a shortage of the hormone insulin (diabetes mellitus) and progressive vision loss due to degeneration of the nerves that carry information from the eyes to the brain (optic atrophy). People with Wolfram syndrome often also have pituitary gland dysfunction that results in the excretion of excessive amounts of urine (diabetes insipidus), hearing loss caused by changes in the inner ear (sensorineural deafness), urinary tract problems, reduced amounts of the sex hormone testosterone in males (hypogonadism), or neurological or psychiatric disorders. Diabetes mellitus is typically the first symptom of Wolfram syndrome, usually diagnosed around age 6. Nearly everyone with Wolfram syndrome who develops diabetes mellitus requires insulin replacement therapy. Optic atrophy is often the next symptom to appear, usually around age 11. The first signs of optic atrophy are loss of color vision and side ( peripheral) vision. Over time, the vision problems get worse, and people with optic atrophy are usually blind within approximately 8 years after signs of optic atrophy first begin. In diabetes insipidus, the pituitary gland, which is located at the base of the brain, does not function normally. This abnormality disrupts the release of a hormone called vasopressin, which helps control the body's water balance and urine production. Approximately 70 percent of people with Wolfram syndrome have diabetes insipidus. Pituitary gland dysfunction can also cause hypogonadism in males. The lack of testosterone that occurs with hypogonadism affects growth and sexual development. -

Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism—Review
molecules Review Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism—Review 1, 1, 1 1 Trnka Daniel y , Hossain Md Faruq y , Jordt Laura Magdalena , Gellert Manuela and Lillig Christopher Horst 2,* 1 Institute for Medical Biochemistry and Molecular Biology, University Medicine, University of Greifswald, 17475 Greifswald, Germany; [email protected] (T.D.); [email protected] (H.M.F.); [email protected] (J.L.M.); [email protected] (G.M.) 2 Christopher Horst Lillig, Institute for Medical Biochemistry and Molecular Biology, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, 17475 Greifswald, Germany * Correspondence: [email protected]; Tel.: +49-3834-865407; Fax: +49-3834-865402 These authors contributed equally to this work. y Academic Editor: Pál Perjési Received: 29 July 2020; Accepted: 22 August 2020; Published: 25 August 2020 Abstract: Glutathione (GSH) was initially identified and characterized for its redox properties and later for its contributions to detoxification reactions. Over the past decade, however, the essential contributions of glutathione to cellular iron metabolism have come more and more into focus. GSH is indispensable in mitochondrial iron-sulfur (FeS) cluster biosynthesis, primarily by co-ligating FeS clusters as a cofactor of the CGFS-type (class II) glutaredoxins (Grxs). GSH is required for the export of the yet to be defined FeS precursor from the mitochondria to the cytosol. In the cytosol, it is an essential cofactor, again of the multi-domain CGFS-type Grxs, master players in cellular iron and FeS trafficking. In this review, we summarize the recent advances and progress in this field. The most urgent open questions are discussed, such as the role of GSH in the export of FeS precursors from mitochondria, the physiological roles of the CGFS-type Grx interactions with BolA-like proteins and the cluster transfer between Grxs and recipient proteins. -

Genomic and Transcriptomic Investigations Into the Feed Efficiency Phenotype of Beef Cattle
Provided by the author(s) and NUI Galway in accordance with publisher policies. Please cite the published version when available. Title Genomic and transcriptomic investigations into the feed efficiency phenotype of beef cattle Author(s) Higgins, Marc Publication Date 2019-03-06 Publisher NUI Galway Item record http://hdl.handle.net/10379/15008 Downloaded 2021-09-25T18:07:39Z Some rights reserved. For more information, please see the item record link above. Genomic and Transcriptomic Investigations into the Feed Efficiency Phenotype of Beef Cattle Marc Higgins, B.Sc., M.Sc. A thesis submitted for the Degree of Doctor of Philosophy to the Discipline of Biochemistry, School of Natural Sciences, National University of Ireland, Galway. Supervisor: Dr. Derek Morris Discipline of Biochemistry, School of Natural Sciences, National University of Ireland, Galway. Supervisor: Dr. Sinéad Waters Teagasc, Animal and Bioscience Research Department, Animal & Grassland Research and Innovation Centre, Teagasc, Grange. Submitted November 2018 Table of Contents Declaration ................................................................................................................ vii Funding .................................................................................................................... viii Acknowledgements .................................................................................................... ix Abstract ...................................................................................................................... -

The NFB Antagonist CDGSH Iron-Sulfur Domain 2 Is a Promising
International Journal of Molecular Sciences Review The NFκB Antagonist CDGSH Iron-Sulfur Domain 2 Is a Promising Target for the Treatment of Neurodegenerative Diseases Woon-Man Kung 1 and Muh-Shi Lin 2,3,4,5,* 1 Department of Exercise and Health Promotion, College of Kinesiology and Health, Chinese Culture University, Taipei 11114, Taiwan; [email protected] 2 Division of Neurosurgery, Department of Surgery, Kuang Tien General Hospital, Taichung 43303, Taiwan 3 Department of Biotechnology and Animal Science, College of Bioresources, National Ilan University, Yilan 26047, Taiwan 4 Department of Biotechnology, College of Medical and Health Care, Hung Kuang University, Taichung 43302, Taiwan 5 Department of Health Business Administration, College of Medical and Health Care, Hung Kuang University, Taichung 43302, Taiwan * Correspondence: [email protected]; Tel.: +886-4-2665-1900 Abstract: Proinflammatory response and mitochondrial dysfunction are related to the pathogenesis of neurodegenerative diseases (NDs). Nuclear factor κB (NFκB) activation has been shown to exaggerate proinflammation and mitochondrial dysfunction, which underlies NDs. CDGSH iron-sulfur domain 2 (CISD2) has been shown to be associated with peroxisome proliferator-activated receptor-β (PPAR- β) to compete for NFκB and antagonize the two aforementioned NFκB-provoked pathogeneses. Therefore, CISD2-based strategies hold promise in the treatment of NDs. CISD2 protein belongs to the human NEET protein family and is encoded by the CISD2 gene (located at 4q24 in humans). In CISD2, the [2Fe-2S] cluster, through coordinates of 3-cysteine-1-histidine on the CDGSH domain, acts as a homeostasis regulator under environmental stress through the transfer of electrons or iron-sulfur clusters. -

WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T (51) International Patent Classification: David [US/US]; 13539 N . 95th Way, Scottsdale, AZ C12Q 1/68 (2006.01) 85260 (US). (21) International Application Number: (74) Agent: AKHAVAN, Ramin; Caris Science, Inc., 6655 N . PCT/US20 12/0425 19 Macarthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 14 June 2012 (14.06.2012) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, English (25) Filing Language: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, Publication Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/497,895 16 June 201 1 (16.06.201 1) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 61/499,138 20 June 201 1 (20.06.201 1) US OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 61/501,680 27 June 201 1 (27.06.201 1) u s SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 61/506,019 8 July 201 1(08.07.201 1) u s TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Fe-S Protein Synthesis in Green Algae Mitochondria
plants Review Fe-S Protein Synthesis in Green Algae Mitochondria Diego F. Gomez-Casati * , Maria V. Busi *, Julieta Barchiesi, Maria A. Pagani , Noelia S. Marchetti-Acosta and Agustina Terenzi Centro de Estudios Fotosintéticos y Bioquímicos (CEFOBI-CONICET), Universidad Nacional de Rosario, 2000 Rosario, Argentina; [email protected] (J.B.); [email protected] (M.A.P.); [email protected] (N.S.M.-A.); [email protected] (A.T.) * Correspondence: [email protected] (D.F.G.-C.); [email protected] (M.V.B.); Tel.: +54-341-4391955 (ext. 113) (D.F.G.-C. & M.V.B.) Abstract: Iron and sulfur are two essential elements for all organisms. These elements form the Fe-S clusters that are present as cofactors in numerous proteins and protein complexes related to key processes in cells, such as respiration and photosynthesis, and participate in numerous enzymatic reactions. In photosynthetic organisms, the ISC and SUF Fe-S cluster synthesis pathways are located in organelles, mitochondria, and chloroplasts, respectively. There is also a third biosynthetic machinery in the cytosol (CIA) that is dependent on the mitochondria for its function. The genes and proteins that participate in these assembly pathways have been described mainly in bacteria, yeasts, humans, and recently in higher plants. However, little is known about the proteins that participate in these processes in algae. This review work is mainly focused on releasing the information on the existence of genes and proteins of green algae (chlorophytes) that could participate in the assembly process of Fe-S groups, especially in the mitochondrial ISC and CIA pathways.