The Ontogeny of Robin Sequence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pfeiffer Syndrome Type II Discovered Perinatally
Diagnostic and Interventional Imaging (2012) 93, 785—789 CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector LETTER / Musculoskeletal imaging Pfeiffer syndrome type II discovered perinatally: Report of an observation and review of the literature a,∗ a a a H. Ben Hamouda , Y. Tlili , S. Ghanmi , H. Soua , b c b a S. Jerbi , M.M. Souissi , H. Hamza , M.T. Sfar a Unité de néonatologie, service de pédiatrie, CHU Tahar Sfar, 5111 Mahdia, Tunisia b Service de radiologie, CHU Tahar Sfar, 5111 Mahdia, Tunisia c Service de gynéco-obstétrique, CHU Tahar Sfar, 5111 Mahdia, Tunisia Pfeiffer syndrome, described for the first time by Pfeiffer in 1964, is a rare hereditary KEYWORDS condition combining osteochondrodysplasia with craniosynostosis [1]. This syndrome is Pfeiffer syndrome; also called acrocephalosyndactyly type 5, which is divided into three sub-types. Type I Cloverleaf skull; is the classic Pfeiffer syndrome, with autosomal dominant transmission, often associated Craniosynostosis; with normal intelligence. Types II and III occur as sporadic cases in individuals who have Syndactyly; craniosynostosis with broad thumbs, broad big toes, ankylosis of the elbows and visceral Prenatal diagnosis abnormalities [2]. We report a case of Pfeiffer syndrome type II, discovered perinatally, which is distinguished from type III by the skull appearing like a cloverleaf, and we shall discuss the clinical, radiological and evolutive features and the advantage of prenatal diagnosis of this syndrome with a review of the literature. Observation The case involved a male premature baby born at 36 weeks of amenorrhoea with multiple deformities at birth. The parents were not blood-related and in good health who had two other boys and a girl with normal morphology. -

Lordosis, Kyphosis, and Scoliosis
SPINAL CURVATURES: LORDOSIS, KYPHOSIS, AND SCOLIOSIS The human spine normally curves to aid in stability or balance and to assist in absorbing shock during movement. These gentle curves can be seen from the side or lateral view of the spine. When viewed from the back, the spine should run straight down the middle of the back. When there are abnormalities or changes in the natural spinal curvature, these abnormalities are named with the following conditions and include the following symptoms. LORDOSIS Some lordosis is normal in the lower portion or, lumbar section, of the human spine. A decreased or exaggerated amount of lordosis that is causing spinal instability is a condition that may affect some patients. Symptoms of Lordosis include: ● Appearance of sway back where the lower back region has a pronounced curve and looks hollow with a pronounced buttock area ● Difficulty with movement in certain directions ● Low back pain KYPHOSIS This condition is diagnosed when the patient has a rounded upper back and the spine is bent over or curved more than 50 degrees. Symptoms of Kyphosis include: ● Curved or hunched upper back ● Patient’s head that leans forward ● May have upper back pain ● Experiences upper back discomfort after movement or exercise SCOLIOSIS The most common of the three curvatures. This condition is diagnosed when the spine looks like a “s” or “c” from the back. The spine is not straight up and down but has a curve or two running side-to-side. Sagittal Balance Definition • Sagittal= front-to-back direction (sagittal plane) • Imbalance= Lack of harmony or balance Etiology • Excessive lordosis (backwards lean) or kyphosis (forward lean) • Traumatic injury • Previous spinal fusion that disrupted sagittal balance Effects • Low back pain • Difficulty walking • Inability to look straight ahead when upright The most ergonomic and natural posture is to maintain neutral balance, with the head positioned over the shoulders and pelvis. -

FROGLOG Newsletter of the Declining Amphibian Populations Task Force
Salamandra salamandra by Franco Andreone ISSN 1026-0269 FROGLOG Newsletter of the Declining Amphibian Populations Task Force August 2004, Number 64. Meteyer et al. (2000) and Ouellet very low number of abnormalities. We (2000). only found one L. kuhlii, which may We examined a total of 4,331 have strayed from a nearby stream. frogs of 23 species and found 20 A third of abnormalities were types of deformities in 9 species of due to trauma; these included digit frogs. We divided deformities into two amputations (16% of all general types: developmental abnormalities), limb amputations (2%), abnormalities and trauma (injuries). fractured limbs (7%) and skin wounds Morphological Abnormalities in We distinguished trauma (4%). The most common Frogs of West Java, Indonesia abnormalities based on the developmental abnormalities were appearance of old scars or, if they digital (43%) and, of these, By Mirza D. Kusrini, Ross A. Alford, involved digits, the occurrence of brachydactyly (16.3%), syndactyly Anisa Fitri, Dede M. Nasir, Sumantri digital re-growth. Developmental (14.6%) and ectrodactyly (11.4%) Rahardyansah abnormalities occurred in limbs were the three most common. In recent decades, amphibian (amelia, micromelia, brachymelia, The oldest specimen of F. deformities have generated public hemimelia, ectromelia, taumelia, cuta- limnocharis stored in the MZB that interest as high incidences have been neous fusions), digits (ectrodactyly, exhibited abnormalities was a juvenile found in several locations, notably in brachydactyly, syndactyly, polydactyly, frog captured on 16 November 1921 North America (Helgen et al., 1998; clinodactyly), the back-bone (scoli- from Bogor without one leg (amelia) Ouellet, 2000). The only report on the osis), the eyes (anophthalmy) and the (ID057.10). -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

SCOLIOSIS: What Is New and True? Natural History, Screening/Evaluation and Treatment
SCOLIOSIS: what is new and true? Natural history, screening/evaluation and treatment Kathleen Moen MD Swedish Pediatric Specialty Update January 24, 2020 OBJECTIVES • 1) review of entity adolescent idiopathic scoliosis • 2) review the natural history of treated and untreated disease • 3) review screening recommendations and delineating patients who warrant referral • 4) review of treatment modalities Scoliosis • Definition: a lateral spinal curvature measuring at least 10 degrees on xray • Complex 3dimensional spinal deformity Adolescent Idiopathic Scoliosis • AIS: Scoliosis most often develops and progresses during the most rapid times of growth, typically between ages 10 ‐15 years. • NOT infantile or juvenile scoliosis which have different natural history profiles • NOT congenital spinal deformities, neuromuscular scoliosis or spinal dysraphism Adolescent Idiopathic Scoliosis • Classic Teaching – Small curves are common – Progression occurs during most rapid times of growth – Female predominance – Big curves get bigger – Etiology unclear: familial predisposition, and? – Idiopathic scoliosis not typically painful Adolescent Idiopathic Scoliosis • Mild scoliosis curves are common, affecting 2‐3% of the adolescent population, males and females equally • Less than 0.1% of adolescents have curves greater than 40 degrees. • Ratio of females to males 10:1 Cobb Angle Female:Male Prevalence >10 1.4‐2 :1 2 ‐ 3 % >20 5:1 .3 ‐ .5 % >30 10:1 .1 ‐ .3 % >40 10:1 <0.1% Weinstein, SL Adolescent Idiopathic Scoliosis, Prevalence and Natural History. Instructional -

Genetics of Congenital Hand Anomalies
G. C. Schwabe1 S. Mundlos2 Genetics of Congenital Hand Anomalies Die Genetik angeborener Handfehlbildungen Original Article Abstract Zusammenfassung Congenital limb malformations exhibit a wide spectrum of phe- Angeborene Handfehlbildungen sind durch ein breites Spektrum notypic manifestations and may occur as an isolated malforma- an phänotypischen Manifestationen gekennzeichnet. Sie treten tion and as part of a syndrome. They are individually rare, but als isolierte Malformation oder als Teil verschiedener Syndrome due to their overall frequency and severity they are of clinical auf. Die einzelnen Formen kongenitaler Handfehlbildungen sind relevance. In recent years, increasing knowledge of the molecu- selten, besitzen aber aufgrund ihrer Häufigkeit insgesamt und lar basis of embryonic development has significantly enhanced der hohen Belastung für Betroffene erhebliche klinische Rele- our understanding of congenital limb malformations. In addi- vanz. Die fortschreitende Erkenntnis über die molekularen Me- tion, genetic studies have revealed the molecular basis of an in- chanismen der Embryonalentwicklung haben in den letzten Jah- creasing number of conditions with primary or secondary limb ren wesentlich dazu beigetragen, die genetischen Ursachen kon- involvement. The molecular findings have led to a regrouping of genitaler Malformationen besser zu verstehen. Der hohe Grad an malformations in genetic terms. However, the establishment of phänotypischer Variabilität kongenitaler Handfehlbildungen er- precise genotype-phenotype correlations for limb malforma- schwert jedoch eine Etablierung präziser Genotyp-Phänotyp- tions is difficult due to the high degree of phenotypic variability. Korrelationen. In diesem Übersichtsartikel präsentieren wir das We present an overview of congenital limb malformations based Spektrum kongenitaler Malformationen, basierend auf einer ent- 85 on an anatomic and genetic concept reflecting recent molecular wicklungsbiologischen, anatomischen und genetischen Klassifi- and developmental insights. -
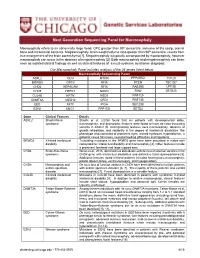
Macrocephaly Information Sheet 6-13-19
Next Generation Sequencing Panel for Macrocephaly Clinical Features: Macrocephaly refers to an abnormally large head, OFC greater than 98th percentile, inclusive of the scalp, cranial bone and intracranial contents. Megalencephaly, brain weight/volume ratio greater than 98th percentile, results from true enlargement of the brain parenchyma [1]. Megalencephaly is typically accompanied by macrocephaly, however macrocephaly can occur in the absence of megalencephaly [2]. Both macrocephaly and megalencephaly can been seen as isolated clinical findings as well as clinical features of a mutli-systemic syndromic diagnosis. Our Macrocephaly Panel includes analysis of the 36 genes listed below. Macrocephaly Sequencing Panel ASXL2 GLI3 MTOR PPP2R5D TCF20 BRWD3 GPC3 NFIA PTEN TBC1D7 CHD4 HEPACAM NFIX RAB39B UPF3B CHD8 HERC1 NONO RIN2 ZBTB20 CUL4B KPTN NSD1 RNF125 DNMT3A MED12 OFD1 RNF135 EED MITF PIGA SEC23B EZH2 MLC1 PPP1CB SETD2 Gene Clinical Features Details ASXL2 Shashi-Pena Shashi et al. (2016) found that six patients with developmental delay, syndrome macrocephaly, and dysmorphic features were found to have de novo truncating variants in ASXL2 [3]. Distinguishing features were macrocephaly, absence of growth retardation, and variability in the degree of intellectual disabilities The phenotype also consisted of prominent eyes, arched eyebrows, hypertelorism, a glabellar nevus flammeus, neonatal feeding difficulties and hypotonia. BRWD3 X-linked intellectual Truncating mutations in the BRWD3 gene have been described in males with disability nonsyndromic intellectual disability and macrocephaly [4]. Other features include a prominent forehead and large cupped ears. CHD4 Sifrim-Hitz-Weiss Weiss et al., 2016, identified five individuals with de novo missense variants in the syndrome CHD4 gene with intellectual disabilities and distinctive facial dysmorphisms [5]. -

Craniofacial Development After Three Different Palatoplasties in Children Born with Isolated Cleft Palate
From the DEPARTMENT OF DENTAL MEDICINE Karolinska Institutet, Stockholm, Sweden CRANIOFACIAL DEVELOPMENT AFTER THREE DIFFERENT PALATOPLASTIES IN CHILDREN BORN WITH ISOLATED CLEFT PALATE Konstantinos A. Parikakis Stockholm 2018 All previously published papers were reproduced with permission from the publisher Published by Karolinska Institutet Printed by Eprint AB 2018 © Konstantinos A. Parikakis, 2018 ISBN 978-91-7831-277-1 Craniofacial development after three different palatoplasties in children born with isolated cleft palate THESIS FOR DOCTORAL DEGREE (Ph.D.) By Konstantinos A. Parikakis Principal Supervisor: Opponent: Associate Professor Agneta Karsten Professor David Rice Karolinska Institutet University of Helsinki Department of Dental Medicine Department of Orthodontics Division of Orthodontics and Pedodontics Examination Board: Co-supervisor(s): Associate Professor Magnus Becker Associate Professor Ola Larson University of Lund Karolinska University Hospital Department of Plastic and Reconstructive Surgery Department of Reconstructive Plastic Surgery Professor Britt Gustafsson Karolinska Institutet Department of Clinical Science, Intervention and Technology (CLINTEC) Division of Pediatrics Associate Professor Farhan Bazargani University of Örebro Centrum för Specialisttandvard Department of Orthodontics To Christina, little Anastasios and…forthcoming Vassilios “Wherever the art of Medicine is loved, there is also a love of Humanity” Hippocrates of Kos, c.460-370 B.C. ABSTRACT Introduction: Different palatoplasties are applied for -

Guidelines for Conducting Birth Defects Surveillance
NATIONAL BIRTH DEFECTS PREVENTION NETWORK HTTP://WWW.NBDPN.ORG Guidelines for Conducting Birth Defects Surveillance Edited By Lowell E. Sever, Ph.D. June 2004 Support for development, production, and distribution of these guidelines was provided by the Birth Defects State Research Partnerships Team, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention Copies of Guidelines for Conducting Birth Defects Surveillance can be viewed or downloaded from the NBDPN website at http://www.nbdpn.org/bdsurveillance.html. Comments and suggestions on this document are welcome. Submit comments to the Surveillance Guidelines and Standards Committee via e-mail at [email protected]. You may also contact a member of the NBDPN Executive Committee by accessing http://www.nbdpn.org and then selecting Network Officers and Committees. Suggested citation according to format of Uniform Requirements for Manuscripts ∗ Submitted to Biomedical Journals:∗ National Birth Defects Prevention Network (NBDPN). Guidelines for Conducting Birth Defects Surveillance. Sever, LE, ed. Atlanta, GA: National Birth Defects Prevention Network, Inc., June 2004. National Birth Defects Prevention Network, Inc. Web site: http://www.nbdpn.org E-mail: [email protected] ∗International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. Ann Intern Med 1988;108:258-265. We gratefully acknowledge the following individuals and organizations who contributed to developing, writing, editing, and producing this document. NBDPN SURVEILLANCE GUIDELINES AND STANDARDS COMMITTEE STEERING GROUP Carol Stanton, Committee Chair (CO) Larry Edmonds (CDC) F. John Meaney (AZ) Glenn Copeland (MI) Lisa Miller-Schalick (MA) Peter Langlois (TX) Leslie O’Leary (CDC) Cara Mai (CDC) EDITOR Lowell E. -

Craniofacial Center
Craniofacial Center The team concept The Craniofacial Center at Children’s Hospital New Orleans is dedicated to providing holistic, coordinated, state-of-the-art care to children with craniofacial differences. All team members specialize in complexities of caring for children with clefts and other craniofacial conditions. Children with clefts and craniofacial differences thrive best when cared for by specialists from many different disciplines. The team approach ensures that healthcare providers work together to implement a single, coordinated, and patient-centered treatment plan unique to your child. Craniofacial Center Craniofacial Pediatrics Genetics Otolaryngology The craniofacial pediatrician will Many babies with craniofacial Our otolaryngologists are surgeons diagnose your child and manage conditions have “isolated” problems with expertise in treating disorders medical problems related to that do not affect their general of the head, neck, ears, nose and their craniofacial differences. The health. The geneticist identifies throat in children of all ages. They physician guides your child’s overall those few patients who may have a assess and monitor your child’s treatment and works with other more complicated genetic condition hearing, ears, feeding, breathing team members to coordinate associated with other medical and speech development. specialty care. Your craniofacial problems and/or family history. They pediatrician will be familiar with all can advise you about the pros and Neurosurgery aspects of your child’s condition and cons of genetic testing, counsel the Neurosurgeons specialize in treating with your family’s needs and desires. family, and give information about children with abnormalities of the The craniofacial pediatrician will the prognosis and recurrence risks. -

CASE REPORT Radiographic Diagnosis of a Rare Case Of
CASE REPORT Radiographic diagnosis of a rare case of oculodentodigital dysplasia Umesh Chandra Parashari, M.D. Sachin Khanduri, M.D. Samarjit Bhadury, M.D. Fareena Akbar Qayyum, M.B.B.S. Department of Radiodiagnosis, Lucknow Medical College, Lucknow, Uttar Pradesh, India Corresponding author: U Parashari ([email protected]) Abstract Oculodentodigital dysplasia (ODDD), also known as oculodento- osseous dysplasia, is an extremely rare autosomal dominant disorder with high penetrance, intra- and interfamilial phenotypic variability, and advanced paternal age in sporadic cases. The incidence of this disease is not precisely known, with only 243 cases reported in the scientific literature, suggesting an incidence of around 1 in 10 million people. It is marked mainly by eye abnormalities, craniofacial dysmorphism, dental anomalies, hand and foot malformations, various skeletal defects, and mildly Fig. 1. Photograph of the patient at age one year (1A) and 16 years (1B and 1C) showing hypotrichosis and pili annulati. The face is small with narrow delayed mental development. Neurological changes may appear eyes, thin nose, prominent columella and wide mandible. The fingers are earlier in each subsequent generation. This case report describes underdeveloped and deformed. a radiological diagnosis of ODDD based on physical appearance, clinical features and radiographic findings in a 16-year-old girl. Case report A 16-year-old girl presented to the hospital with complaints of weakness Introduction in her lower limbs, abnormal dentition and bladder incontinence. On Oculodentodigital dysplasia (ODDD) is a condition that affects many general examination, her gait was ataxic with moderate spasticity in parts of the body, particularly the eyes, teeth and fingers, as the both legs. -

A Guide to Safety Protocols for International Craniofacial Outreach
CE: R.R.; SCS-20-0960; Total nos of Pages: 4; SCS-20-0960 SPECIAL EDITORIAL A Guide to Developing Safety Protocols for International Craniofacial Outreach Programs During the COVID-19 Era Parsa P. Salehi, MD,Ã Adam B. Johnson, MD, PhD,y Brian Rubinstein, MD,z Nima Pahlavan, MD, DDS,§ Babak Azizzadeh, MD, FACS,jj and Usama S. Hamdan, MDô procedures to the ‘‘new normal.’’ One important area of health 07/23/2020 on BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3yRlXg5VZA8ta0m8jqCQrWIIm7WEcSSNRoQmV8QkFTwQ= by https://journals.lww.com/jcraniofacialsurgery from Downloaded Downloaded Abstract: The ongoing COVID-19 outbreak has created obstacles to care delivery that merits attention is the future of craniofacial health care delivery on a global scale. Low- and middle-income outreach programs (CFOP) in the COVID-19 era. from countries (LMICs), many of which already suffered from unmet CFOP provide an essential service to low- and middle-income 1–3 https://journals.lww.com/jcraniofacialsurgery surgical and medical needs, are at great risk of suffering poor health countries (LMICs). Even before the COVID pandemic, the outcomes due to health care access troubles brought on by the surgical needs of LMICs were unmet by existing nongovernmental organizations (NGOs).2 Hence, the pandemic will likely exacerbate pandemic. Craniofacial outreach programs (CFOP)—a staple for 4 craniofacial surgeons—have historically provided essential care to LMICs’ surgical needs. In particular, CFOP are a staple for craniofacial surgeons (which include facial plastic and reconstruc- LMICs. To date, there has not been literature discussing the process of tive surgeons, plastic surgeons, otolaryngologists-head and neck resuming CFOP mission trips.