A Progressive and Complex Clinical Course in Two Family Members With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FROGLOG Newsletter of the Declining Amphibian Populations Task Force
Salamandra salamandra by Franco Andreone ISSN 1026-0269 FROGLOG Newsletter of the Declining Amphibian Populations Task Force August 2004, Number 64. Meteyer et al. (2000) and Ouellet very low number of abnormalities. We (2000). only found one L. kuhlii, which may We examined a total of 4,331 have strayed from a nearby stream. frogs of 23 species and found 20 A third of abnormalities were types of deformities in 9 species of due to trauma; these included digit frogs. We divided deformities into two amputations (16% of all general types: developmental abnormalities), limb amputations (2%), abnormalities and trauma (injuries). fractured limbs (7%) and skin wounds Morphological Abnormalities in We distinguished trauma (4%). The most common Frogs of West Java, Indonesia abnormalities based on the developmental abnormalities were appearance of old scars or, if they digital (43%) and, of these, By Mirza D. Kusrini, Ross A. Alford, involved digits, the occurrence of brachydactyly (16.3%), syndactyly Anisa Fitri, Dede M. Nasir, Sumantri digital re-growth. Developmental (14.6%) and ectrodactyly (11.4%) Rahardyansah abnormalities occurred in limbs were the three most common. In recent decades, amphibian (amelia, micromelia, brachymelia, The oldest specimen of F. deformities have generated public hemimelia, ectromelia, taumelia, cuta- limnocharis stored in the MZB that interest as high incidences have been neous fusions), digits (ectrodactyly, exhibited abnormalities was a juvenile found in several locations, notably in brachydactyly, syndactyly, polydactyly, frog captured on 16 November 1921 North America (Helgen et al., 1998; clinodactyly), the back-bone (scoli- from Bogor without one leg (amelia) Ouellet, 2000). The only report on the osis), the eyes (anophthalmy) and the (ID057.10). -

Genetics of Congenital Hand Anomalies
G. C. Schwabe1 S. Mundlos2 Genetics of Congenital Hand Anomalies Die Genetik angeborener Handfehlbildungen Original Article Abstract Zusammenfassung Congenital limb malformations exhibit a wide spectrum of phe- Angeborene Handfehlbildungen sind durch ein breites Spektrum notypic manifestations and may occur as an isolated malforma- an phänotypischen Manifestationen gekennzeichnet. Sie treten tion and as part of a syndrome. They are individually rare, but als isolierte Malformation oder als Teil verschiedener Syndrome due to their overall frequency and severity they are of clinical auf. Die einzelnen Formen kongenitaler Handfehlbildungen sind relevance. In recent years, increasing knowledge of the molecu- selten, besitzen aber aufgrund ihrer Häufigkeit insgesamt und lar basis of embryonic development has significantly enhanced der hohen Belastung für Betroffene erhebliche klinische Rele- our understanding of congenital limb malformations. In addi- vanz. Die fortschreitende Erkenntnis über die molekularen Me- tion, genetic studies have revealed the molecular basis of an in- chanismen der Embryonalentwicklung haben in den letzten Jah- creasing number of conditions with primary or secondary limb ren wesentlich dazu beigetragen, die genetischen Ursachen kon- involvement. The molecular findings have led to a regrouping of genitaler Malformationen besser zu verstehen. Der hohe Grad an malformations in genetic terms. However, the establishment of phänotypischer Variabilität kongenitaler Handfehlbildungen er- precise genotype-phenotype correlations for limb malforma- schwert jedoch eine Etablierung präziser Genotyp-Phänotyp- tions is difficult due to the high degree of phenotypic variability. Korrelationen. In diesem Übersichtsartikel präsentieren wir das We present an overview of congenital limb malformations based Spektrum kongenitaler Malformationen, basierend auf einer ent- 85 on an anatomic and genetic concept reflecting recent molecular wicklungsbiologischen, anatomischen und genetischen Klassifi- and developmental insights. -

CASE REPORT Radiographic Diagnosis of a Rare Case Of
CASE REPORT Radiographic diagnosis of a rare case of oculodentodigital dysplasia Umesh Chandra Parashari, M.D. Sachin Khanduri, M.D. Samarjit Bhadury, M.D. Fareena Akbar Qayyum, M.B.B.S. Department of Radiodiagnosis, Lucknow Medical College, Lucknow, Uttar Pradesh, India Corresponding author: U Parashari ([email protected]) Abstract Oculodentodigital dysplasia (ODDD), also known as oculodento- osseous dysplasia, is an extremely rare autosomal dominant disorder with high penetrance, intra- and interfamilial phenotypic variability, and advanced paternal age in sporadic cases. The incidence of this disease is not precisely known, with only 243 cases reported in the scientific literature, suggesting an incidence of around 1 in 10 million people. It is marked mainly by eye abnormalities, craniofacial dysmorphism, dental anomalies, hand and foot malformations, various skeletal defects, and mildly Fig. 1. Photograph of the patient at age one year (1A) and 16 years (1B and 1C) showing hypotrichosis and pili annulati. The face is small with narrow delayed mental development. Neurological changes may appear eyes, thin nose, prominent columella and wide mandible. The fingers are earlier in each subsequent generation. This case report describes underdeveloped and deformed. a radiological diagnosis of ODDD based on physical appearance, clinical features and radiographic findings in a 16-year-old girl. Case report A 16-year-old girl presented to the hospital with complaints of weakness Introduction in her lower limbs, abnormal dentition and bladder incontinence. On Oculodentodigital dysplasia (ODDD) is a condition that affects many general examination, her gait was ataxic with moderate spasticity in parts of the body, particularly the eyes, teeth and fingers, as the both legs. -

Current Advances in Holt-Oram Syndrome Taosheng Huang, MD, Phd
Current advances in Holt-Oram syndrome Taosheng Huang, MD, PhD Holt-Oram syndrome is an autosomal-dominant condition Clinical features characterized by congenital cardiac and forelimb anomalies. It Holt and Oram first described this syndrome when they is caused by mutations of the TBX5 gene, a member of the reported on a family with atrial septal defects and con- T-box family that encodes a transcription factor. Molecular genital anomalies of the thumbs [1]. Since then, about studies have demonstrated that mutations predicted to create 200 clinical papers have been published that further de- null alleles cause substantial abnormalities in both the limbs lineate the clinical features of Holt-Oram syndrome and heart, and that missense mutations of TBX5 can produce (HOS). The prevalence of HOS is 1 of 100,000 live distinct phenotypes. One class of missense mutations causes births, and it occurs with wide ethnic and geographic significant cardiac malformations but only minor skeletal distribution. Its clinical manifestations have proved to be abnormalities; others might cause extensive upper limb variable [2,3•,4•], but with complete penetrance. All pa- malformations but less significant cardiac abnormalities. tients with HOS have upper limb anomaly and about Intrafamilial variations of the malformations strongly suggest 85% to 95% have cardiac malformation. On the basis of that genetic background or modifier genes play an important these findings, the criteria for diagnosis include either role in the phenotypic expression of HOS. Efforts to the presence of cardiac malformations, conduction de- understand the intracellular pathway of TBX5 would provide a fects and radial ray abnormalities (or both) in an indi- unique window onto the molecular basis of common vidual, or the presence of radial ray abnormalities with or congenital heart diseases and limb malformations. -

Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

Craniofacial Diseases Caused by Defects in Intracellular Trafficking
G C A T T A C G G C A T genes Review Craniofacial Diseases Caused by Defects in Intracellular Trafficking Chung-Ling Lu and Jinoh Kim * Department of Biomedical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-515-294-3401 Abstract: Cells use membrane-bound carriers to transport cargo molecules like membrane proteins and soluble proteins, to their destinations. Many signaling receptors and ligands are synthesized in the endoplasmic reticulum and are transported to their destinations through intracellular trafficking pathways. Some of the signaling molecules play a critical role in craniofacial morphogenesis. Not surprisingly, variants in the genes encoding intracellular trafficking machinery can cause craniofacial diseases. Despite the fundamental importance of the trafficking pathways in craniofacial morphogen- esis, relatively less emphasis is placed on this topic, thus far. Here, we describe craniofacial diseases caused by lesions in the intracellular trafficking machinery and possible treatment strategies for such diseases. Keywords: craniofacial diseases; intracellular trafficking; secretory pathway; endosome/lysosome targeting; endocytosis 1. Introduction Citation: Lu, C.-L.; Kim, J. Craniofacial malformations are common birth defects that often manifest as part of Craniofacial Diseases Caused by a syndrome. These developmental defects are involved in three-fourths of all congenital Defects in Intracellular Trafficking. defects in humans, affecting the development of the head, face, and neck [1]. Overt cranio- Genes 2021, 12, 726. https://doi.org/ facial malformations include cleft lip with or without cleft palate (CL/P), cleft palate alone 10.3390/genes12050726 (CP), craniosynostosis, microtia, and hemifacial macrosomia, although craniofacial dys- morphism is also common [2]. -

Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017
Appendix 3.1 Birth Defects Descriptions for NBDPN Core, Recommended, and Extended Conditions Updated March 2017 Participating members of the Birth Defects Definitions Group: Lorenzo Botto (UT) John Carey (UT) Cynthia Cassell (CDC) Tiffany Colarusso (CDC) Janet Cragan (CDC) Marcia Feldkamp (UT) Jamie Frias (CDC) Angela Lin (MA) Cara Mai (CDC) Richard Olney (CDC) Carol Stanton (CO) Csaba Siffel (GA) Table of Contents LIST OF BIRTH DEFECTS ................................................................................................................................................. I DETAILED DESCRIPTIONS OF BIRTH DEFECTS ...................................................................................................... 1 FORMAT FOR BIRTH DEFECT DESCRIPTIONS ................................................................................................................................. 1 CENTRAL NERVOUS SYSTEM ....................................................................................................................................... 2 ANENCEPHALY ........................................................................................................................................................................ 2 ENCEPHALOCELE ..................................................................................................................................................................... 3 HOLOPROSENCEPHALY............................................................................................................................................................. -

Syndromes Affecting Ear Nose & Throat
Journal of Analytical & Pharmaceutical Research Syndromes Affecting Ear Nose & Throat Keywords: Throat Review Article genetic disorders; oral manifestations; Ear, Nose, Abbreviations: Volume 5 Issue 4 - 2017 TCS: Treacher Collins Syndrome; RHS: Ramsay Hunt Syndrome; FNP: Facial Nerve Palsy; CHD: Congenital Heart Diseases; AD: Alzheimer’s Diseases; HD: Hirschprung Disease; DS: Down Syndrome; ES: Eagle’s Syndrome; OAV: Department of oral pathology and microbiology, Rajiv Gandhi Oculo-Auriculovertebral; VZV: Varicella Zoster Virus; MPS: University of Health Sciences, India Myofacial Pain Syndrome; FMS: Fibromyalgia Syndrome; NF2: *Corresponding author: Kalpajyoti Bhattacharjee, IntroductionNeurofibromatosis Type I Department of oral pathology and microbiology, Rajiv Gandhi University of Health Sciences, Rajarajeswari Dental College The term syndrome denotes set of signs and symptoms that Email: disease or an increased chance of developing to a particular and Hospital, Bangalore, India, Tel: 8951714933; disease.tend to occurThere together are more and thanreflect 4,000 the presencegenetic disordersof a particular that Received: | Published: constitute head and neck syndromes of which more than 300 May 09, 2017 July 13, 2017 entities involve craniofacial structures [1]. The heritage of the term syndrome is ancient and derived from the Greek word sundrome: sun, syn – together + dromos, a running i.e., “run together”, as the features do. There are of mutated cell are confined to one site and leads to formation of numerous syndromes which involve Ear, Nose, Throat areas with Listmonostotic of Syndromes fibrous dysplasia Affecting [2]. Ear Nose & Throat oral manifestations. The aim of this review is to discuss ear, nose a. Goldenhar syndrome, and throat related syndromes with oral manifestations. b. Frey syndrome, Etiopathogenesis c. -

Treatment Posibilities in Ectromelia
PRIKAZI BOLESNIKA BIBLID: 0370-8179, 135(2007) 5-6, p. 346-351 UDC: 616.7-089:615.8 TREATMENT POSIBILITIES IN ECTROMELIA Dan NEMEŞ, Dan POENARU, Oana BERETEU, Roxana ONOFREI, Elena AMARICAI, Alina TOTOREAN 1”Victor Babeş” University of Medicine and Pharmacology, Timisoara, Romania; 2City Universitary and Emergency Hospital-Rehabilitation and Rheumatology Department, Timisoara, Romania; 3Timis County Universitary and Emergency Hospital-II Orthopaedic and Traumatology Department, Timisoara, Romania; 4Timis County Universitary and Emergency Hospital-II Orthopaedic and Traumatology Department, Rehabilitation Department, Timisoara, Romania SUMMARY Introduction Ectromelia is a congenital abnormality characterised by limb growth disturbances (aplasia or hypoplasia) dur- ing the period from 4th to 8th gestation week. Case outline We present a case of hemimezomelic longitudinal ectromelia of the right upper limb associated with other skel- etal abnormalities, surgically treated. An important role in the management of this case is attributed to the complex rehabili- tation programme done before and after each surgical intervention. Conclusion The aim of the complex therapy is to diminish the permanent invalidity of these patients. Key words: ectromelia; surgical treatment; functional rehabilitation INTRODUCTION months of pregnancy. At the age of two, the patient was admitted to the Children’s Clinic with bronchopneumo- Ectromelia is defined as a congenital development nia, anaemia, nutritional dystrophy and plurimalforma- anomaly characterised by limb growth disturbances tive syndrome, treated for a long period with intramus- (aplasia or hypoplasia) or by limb amputations due to cular antibiotics. amniotic liquid insufficiency [1]. This report describes Examination showed growth retardation (height – a case of ectromelia and tries to analyse the efficiency of 125 centimetres, weight – 32 kilogrammes); macrotia, a complex treatment. -

Hemimelia and Absence of the Peroneal Artery
Journal of Perinatology (2014) 34, 156–158 & 2014 Nature America, Inc. All rights reserved 0743-8346/14 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Hemimelia and absence of the peroneal artery S Huda1, G Sangster2, A Pramanik3, S Sankararaman4, H Tice5 and H Ibrahim3 The arterial patterns of the lower extremities of three patients with congenital absence fibulae (hemimelia) were evaluated to determine whether the relationship existed between the absence of peroneal artery and hemimelia. Computerized tomograph angiography revealed the absence of peroneal artery in all the patients with dysplastic limbs and absent fibula. Journal of Perinatology (2014) 34, 156–158; doi:10.1038/jp.2013.137 Keywords: hemimelia; peroneal artery; angiogensis INTRODUCTION spontaneously in the first year and she received prosthesis for the Hemimelia is the commonest long bone deformity with and right lower extremity deformity. estimated prevalence of 5.7 to 20 cases per 1 million live births.1,2 A range of clinical and radiographic abnormalities has been described in patients with congenital fibular deficiency. These are CASE 2 often associated with limb anomalies in both upper and lower M.J. was a female infant born at 37 weeks to a 29-year-old extremities.3–5 Vascularization of tibia, proximal part of the femur multigravida. There was no history of exposure to teratogens or and the fibula occurs between 4 and 7 weeks of embryogenesis. viral illness during pregnancy. Mother had two prior spontaneous The peroneal artery arises from the posterior tibial artery below abortions, and no family history of congenital anomalies. Prenatal the knee, supplying perforating branches to the lateral and ultrasound showed lumbosacral meningocele and hydrocephalus. -

EUROCAT Syndrome Guide
JRC - Central Registry european surveillance of congenital anomalies EUROCAT Syndrome Guide Definition and Coding of Syndromes Version July 2017 Revised in 2016 by Ingeborg Barisic, approved by the Coding & Classification Committee in 2017: Ester Garne, Diana Wellesley, David Tucker, Jorieke Bergman and Ingeborg Barisic Revised 2008 by Ingeborg Barisic, Helen Dolk and Ester Garne and discussed and approved by the Coding & Classification Committee 2008: Elisa Calzolari, Diana Wellesley, David Tucker, Ingeborg Barisic, Ester Garne The list of syndromes contained in the previous EUROCAT “Guide to the Coding of Eponyms and Syndromes” (Josephine Weatherall, 1979) was revised by Ingeborg Barisic, Helen Dolk, Ester Garne, Claude Stoll and Diana Wellesley at a meeting in London in November 2003. Approved by the members EUROCAT Coding & Classification Committee 2004: Ingeborg Barisic, Elisa Calzolari, Ester Garne, Annukka Ritvanen, Claude Stoll, Diana Wellesley 1 TABLE OF CONTENTS Introduction and Definitions 6 Coding Notes and Explanation of Guide 10 List of conditions to be coded in the syndrome field 13 List of conditions which should not be coded as syndromes 14 Syndromes – monogenic or unknown etiology Aarskog syndrome 18 Acrocephalopolysyndactyly (all types) 19 Alagille syndrome 20 Alport syndrome 21 Angelman syndrome 22 Aniridia-Wilms tumor syndrome, WAGR 23 Apert syndrome 24 Bardet-Biedl syndrome 25 Beckwith-Wiedemann syndrome (EMG syndrome) 26 Blepharophimosis-ptosis syndrome 28 Branchiootorenal syndrome (Melnick-Fraser syndrome) 29 CHARGE -
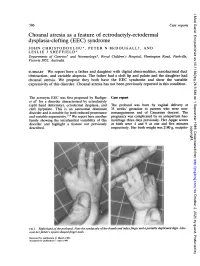
Choanal Atresia As a Feature of Ectrodactyly-Ectodermal Dysplasia
J Med Genet: first published as 10.1136/jmg.26.9.586 on 1 September 1989. Downloaded from 586 Case reports Choanal atresia as a feature of ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome JOHN CHRISTODOULOU*, PETER N McDOUGALLt, AND LESLIE J SHEFFIELD* Departments of Genetics* and Neonatologyt, Royal Children's Hospital, Flemington Road, Parkville, Victoria 3052, Australia. SUMMARY We report here a father and daughter with digital abnormalities, nasolacrimal duct obstruction, and variable alopecia. The father had a cleft lip and palate and the daughter had choanal atresia. We propose they both have the EEC syndrome and show the variable expressivity of this disorder. Choanal atresia has not been previously reported in this condition. The acronym EEC was first proposed by Rudiger Case report et all for a disorder characterised by ectrodactyly (split hand deformity), ectodermal dysplasia, and The proband was born by vaginal delivery at cleft lip/palate. This is an autosomal dominant 35 weeks' gestation to parents who were non- disorder and is notable for both reduced penetrance consanguineous and of Caucasian descent. The and variable expressivity.2- We report here another pregnancy was complicated by an antepartum hae- family showing the intrafamilial variability of this morrhage three days previously. Her Apgar scores disorder and highlight a feature not previously at birth were 4 and 9 at one and five minutes copyright. described. respectively. Her birth weight was 2180 g, occipito- http://jmg.bmj.com/ on October 2, 2021 by guest. Protected FIIRihhnothpoadNtehsnatlyohrtubadnexigeadpatalyupiaediitAs not~ehefte' spoon saedfi ge nal.......... >L..: Received for publication 21 March 1989. Accepted for publication 7 April::.:__1989.