Vinyl Fluoride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS
criteria for a recommended standard . occupational exposure to DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE criteria for a recommended standard... OCCUPATIONAL EXPOSURE TO DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health September 1977 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DHEW (NIOSH) Publication No. 77-193 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and safety of workers exposed to an ever-increasing number of potential hazards at their workplace. The National Institute for Occupational Safety and Health has projected a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the health effects of exposure. The Secretary of Labor will weigh these recommendations along with other considerations such as feasibility and means of implementation in developing regulatory standards. It is intended to present successive reports as research and epidemiologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of the worker. I am pleased to acknowledge the contributions to this report on the decomposition products of fluorocarbon polymers by members of the NIOSH staff and the valuable constructive comments by the Review Consultants on the Decomposition Products of Fluorocarbon Polymers and by Robert B. -

Federal Register/Vol. 84, No. 230/Friday, November 29, 2019
Federal Register / Vol. 84, No. 230 / Friday, November 29, 2019 / Proposed Rules 65739 are operated by a government LIBRARY OF CONGRESS 49966 (Sept. 24, 2019). The Office overseeing a population below 50,000. solicited public comments on a broad Of the impacts we estimate accruing U.S. Copyright Office range of subjects concerning the to grantees or eligible entities, all are administration of the new blanket voluntary and related mostly to an 37 CFR Part 210 compulsory license for digital uses of increase in the number of applications [Docket No. 2019–5] musical works that was created by the prepared and submitted annually for MMA, including regulations regarding competitive grant competitions. Music Modernization Act Implementing notices of license, notices of nonblanket Therefore, we do not believe that the Regulations for the Blanket License for activity, usage reports and adjustments, proposed priorities would significantly Digital Uses and Mechanical Licensing information to be included in the impact small entities beyond the Collective: Extension of Comment mechanical licensing collective’s potential for increasing the likelihood of Period database, database usability, their applying for, and receiving, interoperability, and usage restrictions, competitive grants from the Department. AGENCY: U.S. Copyright Office, Library and the handling of confidential of Congress. information. Paperwork Reduction Act ACTION: Notification of inquiry; To ensure that members of the public The proposed priorities do not extension of comment period. have sufficient time to respond, and to contain any information collection ensure that the Office has the benefit of SUMMARY: The U.S. Copyright Office is requirements. a complete record, the Office is extending the deadline for the extending the deadline for the Intergovernmental Review: This submission of written reply comments program is subject to Executive Order submission of written reply comments in response to its September 24, 2019 to no later than 5:00 p.m. -

1 Draft Chemicals (Management and Safety)
Draft Chemicals (Management and Safety) Rules, 20xx In exercise of the powers conferred by Sections 3, 6 and 25 of the Environment (Protection) Act, 1986 (29 of 1986), and in supersession of the Manufacture, Storage and Import of Hazardous Chemical Rules, 1989 and the Chemical Accidents (Emergency Planning. Preparedness and Response) Rules, 1996, except things done or omitted to be done before such supersession, the Central Government hereby makes the following Rules relating to the management and safety of chemicals, namely: 1. Short Title and Commencement (1) These Rules may be called the Chemicals (Management and Safety) Rules, 20xx. (2) These Rules shall come into force on the date of their publication in the Official Gazette. Chapter I Definitions, Objectives and Scope 2. Definitions (1) In these Rules, unless the context otherwise requires (a) “Act” means the Environment (Protection) Act, 1986 (29 of 1986) as amended from time to time; (b) “Article” means any object whose function is determined by its shape, surface or design to a greater degree than its chemical composition; (c) “Authorised Representative” means a natural or juristic person in India who is authorised by a foreign Manufacturer under Rule 6(2); (d) “Chemical Accident” means an accident involving a sudden or unintended occurrence while handling any Hazardous Chemical, resulting in exposure (continuous, intermittent or repeated) to the Hazardous Chemical causing death or injury to any person or damage to any property, but does not include an accident by reason only -

Vinyl Fluoride
VINYL FLUORIDE This substance was considered by a previous Working Group in February 1995 (IARC, 1995). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature From IARC (1995) and IPCS-CEC (1997) Chem. Abstr. Serv. Reg. No. : 75-02-5 Chem. Abstr. Name : Fluoroethene IUPAC Systematic Name : Fluoroethylene Synonyms : 1-Fluoroethene; 1-fluoroethylene; monofluoroethene; monofluoroethylene RTECS No. : YZ7351000 UN TDG No. : 1860 (stabilized) EINECS No. : 200-832-6 1.1.2 Structural and molecular formulae and relative molecular mass H H C C H F C2H3F Relative molecular mass: 46.04 1.1.3 Chemical and physical properties of the pure substance From IARC (1995), IPCS-CEC (1997), Ebnesajjad (2001) and Lide (2005), unless otherwise specified –459– 460 IARC MONOGRAPHS VOLUME 97 (a) Description : Compressed liquefied gas with characteristic odour; may travel along the ground; distant ignition possible (b) Boiling-point : –72.2 oC (c) Melting-point : –160.5 oC (d) Spectroscopy data : Infrared (prism [30864]; grating [48458P]) and mass [15] spectral data have been reported. (e) Solubility : Slightly soluble in water (15.4 g/L at 6.9 MPa) (f) Vapour pressure : 370 psi [2.553 MPa] at 21 oC (g) Relative vapour density (air = 1) : 1.6 (h) Reactivity : Reacts with alkali and alkaline earth metals, powdered aluminium, zinc and beryllium. (i) Density: 0.636 at 21 °C (j) Stability in water: The HYDROWIN Program (v1.67) cannot estimate a hydrolysis rate constant for this chemical structure; volatilization is a major fate process for vinyl fluoride in water; volatilization half-lives of 2 and 23.5 h have been estimated for a model river (1 m deep) and a model pond (2 m deep), respectively (Lyman et al ., 1990). -

OLCT200 PID Application Note
OLCT200 PID Application note PID Response Factor PID Response Photoionization Detectors (PIDs) respond to a broad variety of organic gases and vapors, and some inorganic compounds. In order to get a response, the photon energy of the lamp must be greater than the ionization energy of the compound. PIDs are available with 10.6 eV lamps. This Technical Article lists the response factors for over 800 compounds with these lamps. The factors presented are inverse to the response, i.e., the higher the number the lower the response. In the table ‘ZR’ means there is no response, NA that the value has not been measured (Not Available), and NV means that the compound is not volatile enough to measure accurately even if its ionization energy is low enough. Isobutylene as Reference Gas It is always most accurate to calibrate a PID directly with the compound to be measured. However, standard gas cylinders of many chemicals are not readily available, and in such cases isobutylene is the standard reference gas used to calibrate. The response of these chemicals differs to isobutylene by the factors listed in the table below. This table allows measurement of any of the compounds in it using only isobutylene for calibration. To obtain the true concentration of the target chemical, multiply the apparent reading by the response factor listed in the table: True Concentration = Apparent Response x Response Factor For example, anisole has a response factor (RF) of 0.47 with a 10.6 eV lamp. Therefore, a reading of 10 ppm using an isobutylene-calibrated unit would correspond to: True Concentration = 10 ppm x 0.47 = 4.7 ppm Most of the Response Factors are pre-programmed into a compound library and can be called up to make the PID read out in units of the compound of interest. -

Chemical Inventory Packet / Calarp Lists / HFD / Dmg 2004 Table 1
HAYWARD FIRE DEPARTMENT CHEMICAL INVENTORY WORKSHEET To prepare a chemical inventory based on the Hayward Fire Code, you are required to report the quantities of chemicals found in the facility, separated by Hazard Category, and by location. You are also to classify the chemicals as to storage and manner of use. Handling and storage requirements are determined by these factors: the nature of the chemical or its hazard; the location; the manner of use or storage; and the quantity involved in each particular manner of use or storage. A Control Area is a building, a portion of the building, or an outside area where chemicals are allowed to be stored, dispensed, used or handled subject to certain conditions and requirements. This packet consists of the following lists and the Chemical Inventory Worksheet form: 1. Hazardous Materials Hazard Categories. This is the list printed on goldenrod paper. Based on the Uniform Fire Code, this is the classification of chemicals by hazard categories. The different hazard categories are defined and examples are provided for each. Most chemicals exhibit more than one hazard, and all hazards should be considered when preparing your chemical inventory. 2. Tables of Regulated Substances Under the CalARP Program. Printed on pink paper, these are the lists of flammable and toxic substances that, if present at or above certain threshold quantities, will require the preparation of a Risk Management Plan 3. Federal List of Extremely Hazardous Substances (EHS). This list is printed on blue paper. These are the substances that the federal government classifies as extremely hazardous substances. The Hayward Fire Department has included “CalARP-Listed or EHS-Listed Chemicals” as a separate Hazard Category. -
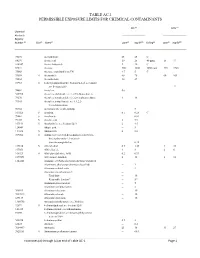
Table Ac-1 Permissible Exposure Limits for Chemical Contaminants
TABLE AC-1 PERMISSIBLE EXPOSURE LIMITS FOR CHEMICAL CONTAMINANTS PEL (d) STEL (o) Chemical Abstracts Registry Number (a) (b) (c) (e) 3(f) (g) (e) 3(f) Skin Name ppm mg/M Ceiling ppm mg/M 75070 Acetaldehyde 25 45 C 64197 Acetic acid 10 25 40 ppm 15 37 108247 Acetic Anhydride 5 20 C 67641 Acetone 500 1200 3000 ppm 750 1780 75868 Acetone cyanohydrin as CN 4.7 5 C 75058 S Acetonitrile 40 70 60 105 98862 Acetophenone 10 49 53963 S 2-Acetylaminofluorene; N-fluoren-2-yl acetamide; see Section 5209 12 74862 Acetylene (h) 540590 Acetylene dichloride; see 1,2-Dichloroethylene 79276 Acetylene tetrabromide:1,1,2,2-tetrabromoethane 1 14 79345 Acetylene tetrachloride; see 1,1,2,2- Tetrachloroethane 50782 Acetylsalicylic acid (Aspirin) 5 107028 S Acrolein 0.1 0.25 C 79061 S Acrylamide -- 0.03 79107 S Acrylic acid 2 5.9 107131 S Acrylonitrile; see Section 5213 2 4.5 124049 Adipic acid -- 5 111693 S Adiponitrile 2 8.8 309002 S Aldrin; 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a- hexahydro-endo-1,2-exo-5,8- dimethanonaphthalene -- 0.25 107186 S Allyl alcohol 0.5 1.25 4 10 107051 Allyl chloride 1 3 2 6 106923 S Allyl glycidyl ether; AGE 0.2 0.93 2179591 Allyl propyl disulfide 2 12 3 18 1344281 Alumina; see Particulates not otherwise regulated Aluminum, alkyls (not otherwise classified) -- 2 Aluminum soluble salts -- 2 Aluminum metal and oxide -- Total dust -- 10 Respirable fraction(n) -- 5(n) Aluminum pyro powders -- 5 Aluminum welding fumes -- 5 300925 Aluminum distearate -- 10 7047849 Aluminum stearate -- 10 637127 Aluminum tristearate -- 10 1300738 Aminodimethylbenzene; -

CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry 3M MN04855110
CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry BY H. G. BRYCE f~Iinnesota ~Vlining and Manufacturing Company, St. Paul, Minnesota I. Introduction ....................................................... 297 II. Historical and Economic Factors ..................................... 298 III. Characteristic Properties of Fluorocarbons .............................. 302 A. Bond Energies and Bond Distances ............................... 302 B. Boiling Points and Melting Points ................................ 303 C. Surface Energy ................................................ 304 D. Stability ...................................................... 309 E. Electrical Properties ............................................ 312 F. Polarity of Oxides or Nitrides and Effect of Structure ............... 312 G. Solubility ..................................................... 314 IV. Refrigerants and Propellants ......................................... 316 A. Properties ..................................................... 317 B. Refrigeration Characteristics ..................................... 320 C. Applications for Specific Compounds ............................. 323 V. Heat Transfer Media ................................................ 323 A. Heat Transfer Processes ........................................ 323 B. Miniaturization ................................................ 324 C. Application of FIuorocarbon Fluids to Heat Transfer Problems ....... 325 D. Properties of Fluorocarbon Fluids ............................... -

Refrigerants
CHAPTER 19 REFRIGERANTS Phaseout of Refrigerants .............................................................................................................. 19.1 Refrigerant Properties .................................................................................................................. 19.4 Refrigerant Performance ............................................................................................................. 19.6 Safety ............................................................................................................................................ 19.6 Leak Detection ............................................................................................................................. 19.7 Effect on Construction Materials ................................................................................................ 19.11 EFRIGERANTS are the working fluids in refrigeration, air- Transport properties of thermal conductivity and viscosity affect Rconditioning, and heat pumping systems. They absorb heat the performance of heat exchangers and piping. High thermal con- from one area, such as an air-conditioned space, and reject it into ductivity and low viscosity are desirable. another, such as outdoors, usually through evaporation and conden- No single fluid satisfies all the attributes desired of a refrigerant; sation, respectively. These phase changes occur both in absorption as a result, a variety of refrigerants is used. This chapter describes and mechanical vapor compression systems, but -

Vinyl Fluoride Resistant to Weather and Have Great Strength, Chemical Inertness, and CAS No
SUBSTANCE PROFILES Vinyl Fluoride resistant to weather and have great strength, chemical inertness, and CAS No. 75-02-5 low permeability to air and water. Polyvinyl fluoride is laminated with aluminum, galvanized steel, and cellulose materials and is used Reasonably anticipated to be a human carcinogen as a protective surface for the exteriors of residential and commercial First Listed in the Tenth Report on Carcinogens (2002) buildings. Polyvinyl fluoride laminated with various plastics has been H used to cover walls, pipes, and electrical equipment and inside aircraft C cabins (IARC 1995). H2C F Carcinogenicity Production Vinyl fluoride is reasonably anticipated to be a human carcinogen based Vinyl fluoride was first prepared in the early 1900s by reaction of zinc on sufficient evidence of carcinogenicity in experimental animals. Both with 1,1-difluoro-2-bromoethane. Modern preparation of vinyl male and female rats exposed to vinyl fluoride by inhalation showed fluoride involves reaction of acetylene and hydrogen fluoride in the increased incidences of hepatic hemangiosarcoma, hepatocellular presence of a mercury-based or aluminum-based catalyst (IARC 1995). adenoma or carcinoma, and Zymbal gland carcinoma. Both male and Annual U.S. production is approximately 3.3 million lb (HSDB female mice exposed to vinyl fluoride by inhalation showed increased 2001). The U.S. Environmental Protection Agency (EPA), through the incidences of hepatic hemangiosarcoma, bronchiolar-alveolar adenoma Office of Pollution Prevention and Toxics, listed vinyl fluoride in the or adenocarcinoma, hepatocellular adenoma, and harderian gland high production volume chemical list in 1990, indicating that annual adenoma. Female mice also showed an increased incidence of production exceeded 1 million lb (EPA 1990). -
C1-C4 Halogenated HC Info 16FEB2018.Xlsx
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) Feb 28, 2018 Administrative Council Mtg - Printed 2/16/20184:11 PM Reported Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR, mfr literature) TURA TSCA CDR Chemical CAS/Chemica chemical name chemical fire 2015 Chemical Name Pesti- blowing feedstock / supressant / propell- Inventory? TRI? List l Number (synonyms) formula Solvent refriger-ant etchant Other Tier II cide agent intermed-iate flame ant status* retardant insulation for refrigerators, freezers, commercial refrigeration equipment, refrigerated containers and LNG ships; spray foam insulation; insulated metal panels; slabstock and molded flexible foam; refrigerant for chillers; and solvents for metal cleaning and electronics, and circuit flush. https://www.honeywell-blowingagents.com/?document=solstice-lba-technical- HFO-1233zd (E) brochure&download=1 Hydro-fluoro-olefin C H ClF YYY yhttps://www.honeywell-refrigerants.com/americas/product/solstice-zd/ (HFO) 3 2 3 CDR 2016 Honeywell: 100% as propellants and blowing agents for plastics product mfg; R-1233zd CDR Honeywell Solstice® ZD refrigerant, Solstice® 1233zdE, Solstice Blowing Agent; Arkema (current Forane® 1233zd blowing agent for spray PU foam, appliance insulation, etc. and solvent (may Honeywell not be available in US) product) C1-C4 Cat 102687-65-0 1-Chloro-3,3,3-trifluoropropene Y 106‐95‐6 Allyl bromide 2-Propenyl bromide C3H5Br Y y Manufacture of synthetic perfumes, other allyl compounds; Insecticidal fumigant; 1-Propene, 3-bromo- Chemical intermediate in organic synthesis, for resins (copolymer with sulfur dioxide) and fragrances; As a fumigant (if quite volatile) or as a contact poison; C1-C4 Cat Used in the manufacture of plastics and dyestuff. -

NJAC 7:1E DISCHARGES of PETROLEUM and OTHER HAZARDOUS SUBSTANCES RULES Statutory Authority
This is a courtesy copy of this rule. All of the Department's rules are compiled in Title 7 of the New Jersey Administrative Code. N.J.A.C. 7:1E DISCHARGES OF PETROLEUM AND OTHER HAZARDOUS SUBSTANCES RULES Statutory authority: N.J.S.A. 58:10-23.11, N.J.S.A. 58:10-46 to 50, N.J.S.A. 13:1K-1 et seq., and N.J.S.A.13:1D-125 through 133 Date last adopted: January 27, 2014 Date last amended: June 1, 2020 For regulatory history and effective dates, see the New Jersey Administrative Code TABLE OF CONTENTS APPENDIX A - LIST OF HAZARDOUS SUBSTANCES Alphabetical Order CAS Number Order 1 This is a courtesy copy of this rule. All of the Department's rules are compiled in Title 7 of the New Jersey Administrative Code. APPENDIX A List of Hazardous Substances (Alphabetical Order) Name CAS Number --------------------------------------------------- ---------------- Abamectin 71751-41-2 Acenaphthene 83-32-9 Acenaphthylene 208-96-8 Acephate 30560-19-1 Acetaldehyde 75-07-0 Acetamide 60-35-5 Acetic acid 64-19-7 Acetic anhydride 108-24-7 Acetone 67-64-1 Acetone cyanohydrin 75-86-5 Acetone thiosemicarbazide 1752-30-3 Acetonitrile 75-05-8 Acetophenone 98-86-2 Acetoxytriphenylstannane 900-95-8 2-Acetylaminofluorene 53-96-3 Acetyl bromide 506-96-7 Acetyl chloride 75-36-5 Acetylene* 74-86-2 1-Acetyl-2-thiourea 591-08-2 Acifluorfen, sodium salt 62476-59-9 Acrolein 107-02-8 Acrylamide 79-06-1 Acrylic acid 79-10-7 Acrylonitrile 107-13-1 Acrylyl chloride 814-68-6 Adipic acid 124-04-9 Adiponitrile 111-69-3 Alachlor 15972-60-8 Aldicarb 116-06-3 Aldicarb sulfone 1646-88-4 Aldrin 309-00-2 d-trans-Allethrin 28057-48-9 Allyl alcohol 107-18-6 Allyl amine 107-11-9 Allyl chloride 107-05-1 Aluminum (fume or dust) 7429-90-5 Aluminum oxide (fibrous forms) 1344-28-1 Aluminum phosphide 20859-73-8 Aluminum sulfate 10043-01-3 *In accordance with N.J.A.C.