Vinyl Fluoride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Vinyl Bromide
FINAL Report on Carcinogens Background Document for Vinyl Bromide Meeting of the NTP Board of Scientific Counselors Report on Carcinogens Subcommittee Prepared for the: U.S. Department of Health and Human Services Public Health Services National Toxicology Program Research Triangle Park, NC 27709 Prepared by: Technology Planning and Management Corporation Canterbury Hall, Suite 310 4815 Emperor Blvd Durham, NC 27703 Contract Number NOI-ES-85421 RoC Background Document for Vinyl Bromide Criteria for Listing Agents, Substances or Mixtures in the Report on Carcinogens US Department of Health and Human Services National Toxicology Program Known to be Human Carcinogens: There is sufficient evidence of carcinogenicity from studies in humans which indicates a causal relationship between exposure to the agent, substance or mixture and human cancer. Reasonably Anticipated to be Human Carcinogens: There is limited evidence of carcinogenicity from studies in humans which indicates that causal interpretation is credible but that alternative explanations such as chance, bias or confounding factors could not adequately be excluded; or There is sufficient evidence of carcinogenicity from studies in experimental animals which indicates there is an increased incidence of malignant and/or a combination of malignant and benign tumors: (1) in multiple species, or at multiple tissue sites, or (2) by multiple routes of exposure, or (3) to an unusual degree with regard to incidence, site or type of tumor or age at onset; or There is less than sufficient evidence of carcinogenicity in humans or laboratory animals, however; the agent, substance or mixture belongs to a well defined, structurally-related class of substances whose members are listed in a previous Report on Carcinogens as either a known to be human carcinogen, or reasonably anticipated to be human carcinogen or there is convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans. -

ETHYLENE DICHLORIDE (L,Z-Dichloroethane) April 19, 1978
NlaSM e~ 9~ 8tdtetue ~~-B~ 19tAwe3() In 1978 CONTENTS NO. TITLE DATE 19 - Z,4-DIAMINOANISOLE (4-Methoxy-m-Phenylenediamine) IN HAIR AND FUR DYES January 13, 1978 «( » 2.0 - TETRACHLOROETHYLENE (perchloroethylene) January 20, 1978 ZI - TRlMELUTIC ANHYDRIDE (TMA) February 3, 1978 ZZ - ETHYLENE THIOUREA April 11, 1978 Z3 - ETHYLENE DIBROMIDE AND DISULFIRAM TOXIC INTERACTION April 11, 1978 ( 4: 1) Z4 - DIRECT BLUE 6, DIRECT BLACK 38, DIRECT BROWN 95 Benzidine Derived Dyes April 17, 1978 Z5 - ETHYLENE DICHLORIDE (l,Z-dichloroethane) April 19, 1978 Z6 - NIAX· Catalyst ESN •••a mixture of Dimethylaminopropionitrile and Bis[Z-(dimethylamino)ethylj ether May 22,1978 27 - CHLOROETHANES: REVIEW OF TOXICITY August 21, 1978 28 - VINYL HALIDES CARCINOGENICITY Vinyl Bromide, Vinyl Chloride, Vinylidene Chloride September 21,1978 (1 _:"_ :3) 29 - GL YCIDYL ETHERS October lZ, 1978 (1 ~7 ) 30 - EPICHLOROHYDRIN October 12, 1978 ( 1~1 ) Cumulative List of NIOSH Current Intelligence Bulletins #l through ;¥o30 for 1975 through 1978 ----------.--------- U. S. DEPARTMENT OF HEALTH, EDUCATION" A /IYID WELFARE Public Health Ser"ice Center tor Disease Control National Institute tor Occupational Satety and Heallf:hiJ / / NIOSH CURRENT INTELLIGENCE BULLETIN REPRINTS - BULLETINS 19 thru 30 for 1978 Leonard J. Bah1man, Editor Technical Evaluation and Review Branch U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health Office of Extramural Coordination and Special Projects Rockville, Maryland 20857 September 1979 DISCLAIMER Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. -

DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS
criteria for a recommended standard . occupational exposure to DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE criteria for a recommended standard... OCCUPATIONAL EXPOSURE TO DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health September 1977 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DHEW (NIOSH) Publication No. 77-193 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and safety of workers exposed to an ever-increasing number of potential hazards at their workplace. The National Institute for Occupational Safety and Health has projected a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the health effects of exposure. The Secretary of Labor will weigh these recommendations along with other considerations such as feasibility and means of implementation in developing regulatory standards. It is intended to present successive reports as research and epidemiologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of the worker. I am pleased to acknowledge the contributions to this report on the decomposition products of fluorocarbon polymers by members of the NIOSH staff and the valuable constructive comments by the Review Consultants on the Decomposition Products of Fluorocarbon Polymers and by Robert B. -

Federal Register/Vol. 84, No. 230/Friday, November 29, 2019
Federal Register / Vol. 84, No. 230 / Friday, November 29, 2019 / Proposed Rules 65739 are operated by a government LIBRARY OF CONGRESS 49966 (Sept. 24, 2019). The Office overseeing a population below 50,000. solicited public comments on a broad Of the impacts we estimate accruing U.S. Copyright Office range of subjects concerning the to grantees or eligible entities, all are administration of the new blanket voluntary and related mostly to an 37 CFR Part 210 compulsory license for digital uses of increase in the number of applications [Docket No. 2019–5] musical works that was created by the prepared and submitted annually for MMA, including regulations regarding competitive grant competitions. Music Modernization Act Implementing notices of license, notices of nonblanket Therefore, we do not believe that the Regulations for the Blanket License for activity, usage reports and adjustments, proposed priorities would significantly Digital Uses and Mechanical Licensing information to be included in the impact small entities beyond the Collective: Extension of Comment mechanical licensing collective’s potential for increasing the likelihood of Period database, database usability, their applying for, and receiving, interoperability, and usage restrictions, competitive grants from the Department. AGENCY: U.S. Copyright Office, Library and the handling of confidential of Congress. information. Paperwork Reduction Act ACTION: Notification of inquiry; To ensure that members of the public The proposed priorities do not extension of comment period. have sufficient time to respond, and to contain any information collection ensure that the Office has the benefit of SUMMARY: The U.S. Copyright Office is requirements. a complete record, the Office is extending the deadline for the extending the deadline for the Intergovernmental Review: This submission of written reply comments program is subject to Executive Order submission of written reply comments in response to its September 24, 2019 to no later than 5:00 p.m. -

Anaerobic Biodegradation of Ethylene Dibromide and 1,2
Clemson University TigerPrints All Dissertations Dissertations 5-2008 ANAEROBIC BIODEGRADATION OF ETHYLENE DIBROMIDE AND 1,2-DICHLOROETHANE IN THE PRESENCE OF FUEL HYDROCARBONS James Henderson Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Environmental Engineering Commons Recommended Citation Henderson, James, "ANAEROBIC BIODEGRADATION OF ETHYLENE DIBROMIDE AND 1,2-DICHLOROETHANE IN THE PRESENCE OF FUEL HYDROCARBONS" (2008). All Dissertations. 213. https://tigerprints.clemson.edu/all_dissertations/213 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. ANAEROBIC BIODEGRADATION OF ETHYLENE DIBROMIDE AND 1,2- DICHLOROETHANE IN THE PRESENCE OF FUEL HYDROCARBONS A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Environmental Engineering and Science by James K. Henderson May 2008 Accepted by: Dr. Ronald W. Falta, Committee Chair Dr. David L. Freedman Dr. Larry W. Murdoch Mr. Stephen H. Shoemaker Dr. Yanru Yang ABSTRACT Field evidence from underground storage tank (UST) sites where leaded gasoline leaked indicates the lead scavengers 1,2-dibromoethane (ethylene dibromide, or EDB) and 1,2-dichloroethane (1,2-DCA) may be present in groundwater at levels that pose unacceptable risk. These compounds are seldom tested for at UST sites. Although dehalogenation of EDB and 1,2-DCA is known to occur, the effect of fuel hydrocarbons on their biodegradability under anaerobic conditions is poorly understood. -

1 Draft Chemicals (Management and Safety)
Draft Chemicals (Management and Safety) Rules, 20xx In exercise of the powers conferred by Sections 3, 6 and 25 of the Environment (Protection) Act, 1986 (29 of 1986), and in supersession of the Manufacture, Storage and Import of Hazardous Chemical Rules, 1989 and the Chemical Accidents (Emergency Planning. Preparedness and Response) Rules, 1996, except things done or omitted to be done before such supersession, the Central Government hereby makes the following Rules relating to the management and safety of chemicals, namely: 1. Short Title and Commencement (1) These Rules may be called the Chemicals (Management and Safety) Rules, 20xx. (2) These Rules shall come into force on the date of their publication in the Official Gazette. Chapter I Definitions, Objectives and Scope 2. Definitions (1) In these Rules, unless the context otherwise requires (a) “Act” means the Environment (Protection) Act, 1986 (29 of 1986) as amended from time to time; (b) “Article” means any object whose function is determined by its shape, surface or design to a greater degree than its chemical composition; (c) “Authorised Representative” means a natural or juristic person in India who is authorised by a foreign Manufacturer under Rule 6(2); (d) “Chemical Accident” means an accident involving a sudden or unintended occurrence while handling any Hazardous Chemical, resulting in exposure (continuous, intermittent or repeated) to the Hazardous Chemical causing death or injury to any person or damage to any property, but does not include an accident by reason only -

OLCT200 PID Application Note
OLCT200 PID Application note PID Response Factor PID Response Photoionization Detectors (PIDs) respond to a broad variety of organic gases and vapors, and some inorganic compounds. In order to get a response, the photon energy of the lamp must be greater than the ionization energy of the compound. PIDs are available with 10.6 eV lamps. This Technical Article lists the response factors for over 800 compounds with these lamps. The factors presented are inverse to the response, i.e., the higher the number the lower the response. In the table ‘ZR’ means there is no response, NA that the value has not been measured (Not Available), and NV means that the compound is not volatile enough to measure accurately even if its ionization energy is low enough. Isobutylene as Reference Gas It is always most accurate to calibrate a PID directly with the compound to be measured. However, standard gas cylinders of many chemicals are not readily available, and in such cases isobutylene is the standard reference gas used to calibrate. The response of these chemicals differs to isobutylene by the factors listed in the table below. This table allows measurement of any of the compounds in it using only isobutylene for calibration. To obtain the true concentration of the target chemical, multiply the apparent reading by the response factor listed in the table: True Concentration = Apparent Response x Response Factor For example, anisole has a response factor (RF) of 0.47 with a 10.6 eV lamp. Therefore, a reading of 10 ppm using an isobutylene-calibrated unit would correspond to: True Concentration = 10 ppm x 0.47 = 4.7 ppm Most of the Response Factors are pre-programmed into a compound library and can be called up to make the PID read out in units of the compound of interest. -

Vinyl Chloride
TOXICOLOGICAL PROFILE FOR VINYL CHLORIDE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry July 2006 VINYL CHLORIDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. VINYL CHLORIDE iii UPDATE STATEMENT A Toxicological Profile for Vinyl Chloride, Draft for Public Comment was released in 2004. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 VINYL CHLORIDE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance’s toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. -

Chemical Inventory Packet / Calarp Lists / HFD / Dmg 2004 Table 1
HAYWARD FIRE DEPARTMENT CHEMICAL INVENTORY WORKSHEET To prepare a chemical inventory based on the Hayward Fire Code, you are required to report the quantities of chemicals found in the facility, separated by Hazard Category, and by location. You are also to classify the chemicals as to storage and manner of use. Handling and storage requirements are determined by these factors: the nature of the chemical or its hazard; the location; the manner of use or storage; and the quantity involved in each particular manner of use or storage. A Control Area is a building, a portion of the building, or an outside area where chemicals are allowed to be stored, dispensed, used or handled subject to certain conditions and requirements. This packet consists of the following lists and the Chemical Inventory Worksheet form: 1. Hazardous Materials Hazard Categories. This is the list printed on goldenrod paper. Based on the Uniform Fire Code, this is the classification of chemicals by hazard categories. The different hazard categories are defined and examples are provided for each. Most chemicals exhibit more than one hazard, and all hazards should be considered when preparing your chemical inventory. 2. Tables of Regulated Substances Under the CalARP Program. Printed on pink paper, these are the lists of flammable and toxic substances that, if present at or above certain threshold quantities, will require the preparation of a Risk Management Plan 3. Federal List of Extremely Hazardous Substances (EHS). This list is printed on blue paper. These are the substances that the federal government classifies as extremely hazardous substances. The Hayward Fire Department has included “CalARP-Listed or EHS-Listed Chemicals” as a separate Hazard Category. -

Total Syntheses of Ageladine A; Part
The Pennsylvania State University The Graduate School Department of Chemistry PART ONE: TOTAL SYNTHESES OF AGELADINE A; PART TWO: STUDIES DIRECTED TOWARDS A TOTAL SYNTHESIS OF ACTINOPHYLLIC ACID A Dissertation in Chemistry by Matthew L. Meketa © 2008 Matthew L. Meketa Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2008 The dissertation of Matthew L. Meketa was reviewed and approved* by the following: Steven M. Weinreb Russell and Mildred Marker Professor of Natural Products Chemistry Dissertation Advisor Chair of Committee Raymond L. Funk Professor of Chemistry Scott T. Phillips Assistant Professor of Chemistry Caroline E. Clifford Senior Research Associate Ayusman Sen Professor of Chemistry Head of the Department of Chemistry * Signatures are on file in the Graduate School ii Abstract Part One We have completed two unique total syntheses of the marine natural product ageladine A (1). Our first generation total synthesis of this angiogenesis-inhibitory marine metabolite features a 6π-1-azatriene electrocyclization of triene 63 to form the pyridine ring of intermediate 66. A subsequent Suzuki-Miyaura coupling of N-Boc- pyrrole-2-boronic acid 67 with a chloroimidazopyridine 82 furnished tricycle 88. Unfortunately, the dibromination of pyrrole 88 to give the natural product proved to be difficult to control, due to the high reactivity of this intermediate to various brominating conditions. After some experimentation, however, the optimal conditions found for the halogenation was to treat pyrrole 88 with Br2 in an acetic acid/methanol solvent mixture at 0 ºC to give ageladine A in 17% yield. The assessment of the biological activity of a variety of synthetic structural analogues of ageladine A prepared during this synthesis is described. -
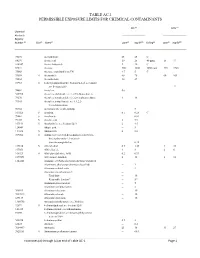
Table Ac-1 Permissible Exposure Limits for Chemical Contaminants
TABLE AC-1 PERMISSIBLE EXPOSURE LIMITS FOR CHEMICAL CONTAMINANTS PEL (d) STEL (o) Chemical Abstracts Registry Number (a) (b) (c) (e) 3(f) (g) (e) 3(f) Skin Name ppm mg/M Ceiling ppm mg/M 75070 Acetaldehyde 25 45 C 64197 Acetic acid 10 25 40 ppm 15 37 108247 Acetic Anhydride 5 20 C 67641 Acetone 500 1200 3000 ppm 750 1780 75868 Acetone cyanohydrin as CN 4.7 5 C 75058 S Acetonitrile 40 70 60 105 98862 Acetophenone 10 49 53963 S 2-Acetylaminofluorene; N-fluoren-2-yl acetamide; see Section 5209 12 74862 Acetylene (h) 540590 Acetylene dichloride; see 1,2-Dichloroethylene 79276 Acetylene tetrabromide:1,1,2,2-tetrabromoethane 1 14 79345 Acetylene tetrachloride; see 1,1,2,2- Tetrachloroethane 50782 Acetylsalicylic acid (Aspirin) 5 107028 S Acrolein 0.1 0.25 C 79061 S Acrylamide -- 0.03 79107 S Acrylic acid 2 5.9 107131 S Acrylonitrile; see Section 5213 2 4.5 124049 Adipic acid -- 5 111693 S Adiponitrile 2 8.8 309002 S Aldrin; 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a- hexahydro-endo-1,2-exo-5,8- dimethanonaphthalene -- 0.25 107186 S Allyl alcohol 0.5 1.25 4 10 107051 Allyl chloride 1 3 2 6 106923 S Allyl glycidyl ether; AGE 0.2 0.93 2179591 Allyl propyl disulfide 2 12 3 18 1344281 Alumina; see Particulates not otherwise regulated Aluminum, alkyls (not otherwise classified) -- 2 Aluminum soluble salts -- 2 Aluminum metal and oxide -- Total dust -- 10 Respirable fraction(n) -- 5(n) Aluminum pyro powders -- 5 Aluminum welding fumes -- 5 300925 Aluminum distearate -- 10 7047849 Aluminum stearate -- 10 637127 Aluminum tristearate -- 10 1300738 Aminodimethylbenzene;