C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) DRAFT - Do Not Cite Or Distribute 10/13/20172:39 PM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1, 1, 1, 3, 3-Pentafluoropropane (HFC-245Fa) (CAS No. 460-73-1)
1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) (CAS No. 460-73-1) JACC Report No. 44 Brussels, June 2004 1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) ECETOC JACC REPORT No. 44 © Copyright - ECETOC European Centre for Ecotoxicology and Toxicology of Chemicals 4 Avenue E. Van Nieuwenhuyse (Bte 6), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC JACC No. 44 1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) 1,1,1,3,3-Pentafluoropropane (HFC-245fa) (CAS No. 460-73-1) CONTENTS EXECUTIVE SUMMARY 1 THE ECETOC SCHEME FOR THE JOINT ASSESSMENT OF COMMODITY CHEMICALS 2 1. SUMMARY AND CONCLUSIONS 3 2. IDENTITY, PHYSICAL, AND CHEMICAL PROPERTIES, ANALYTICAL METHODS 5 2.1 Identity 5 2.2 EC classification and labelling 5 2.3 Physical and chemical properties 5 2.4 Conversion factors 6 2.5 Analytical methods 7 2.5.1 In air 7 2.5.2 In water 7 3. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

United States Patent (19) 11, 3,977,878 Roteman (45) Aug
United States Patent (19) 11, 3,977,878 Roteman (45) Aug. 31, 1976 54 EPOXY RESEN PHOTORESIST WITH 3,522, 194 7/1970 Hada et al........................ 260/47 A ODOFORMAND BISMUTH TRPHENYL 3,708,296 1 / 1973 Schlesinger........................ 96/115 P 3,711,391 lf 1973 Feinberg............................ 96/115 P 75 Inventor: Jerome Roteman, Solon, Ohio 3,720,634 3/1973 Statton ........................... 260/47 EC 73) Assignee: American Can Company, 3,721,617 3/1973 Watt ................................. 260/2 EP Greenwich, Conn. Primary Examiner-Jack P. Brammer 22 Filed: Feb. 26, 1975 Attorney, Agent, or Firm-Robert P. Auber; Ernestine (21) Appl. No.: 553,262 C. Bartlett; George P. Ziehmer Related U.S. Application Data 57 ABSTRACT 62 Division of Ser. No. 369,086, June 1 1, 1973, Pat. No. 3,895,954. Photopolymerizable compositions and processes for photopolymerizing such compositions are provided, 52 U.S. Cl................................ 96/86 P; 96/115 R; said process comprising admixing with said epoxides, 96/ 15 P; 204/159. 18; 204/159.23; photosensitive organohalogen compounds in combina 204/159.24; 96/85; 96/87 R tion with an organometallic compound and thereafter 51 Int. Cl.’....................... G03C 1/94; G03C 1/68 applying energy to the resulting mixture. The or 58 Field of Search............. 96/115 R, 1 15 P, 86 P, ganohalogens decompose to liberate an active catalyst 96/85, 87 R; 204/159.18, 159.23, 159.24; which then serves to initiate polymerization of the ep 260/2 EP, 2 EC, 47 A, 47 C; 427/43 oxide material. The organometallic compound func tions synergistically with the organohalogen to en 56) References Cited hance the film forming properties of the resulting pol UNITED STATES PATENTS ymer and or sensitivity of the polymerizable system. -

PAPERS READ BEFORE the CHEMICAL SOCIETY. XXII1.-On
View Article Online / Journal Homepage / Table of Contents for this issue 773 PAPERS READ BEFORE THE CHEMICAL SOCIETY. XXII1.-On Tetrabromide of Carbon. No. II. By THOMASBOLAS and CHARLESE. GROVES. IN a former paper* we described several methods for the preparation of the hitherto unknown tetrabromide of carbon, and in the present communication we desire to lay before the Society the results of our more recent experiments. In addition to those methods of obtaining the carbon tetrabromide, which we have already published, the fol- lowing are of interest, either from a theoretical point of view, or as affording advantageous means for the preparation of that substance. Action of Bromine on Carbon Disulphide. Our former statement that? bromine had no action on carbon disul- phide requires some modification, as we find that when it is heated to 180" or 200" for several hundred hours with bromine free from both chlorine and iodine, and the contents of the tubes are neutralised and distilled in the usual way, a liquid is obtained, which consists almost entirely of unaltered carbon disulphide ; but when this is allowed to evaporate spontaneously, a small quantity of a crystalline substance is left, which has the appearance and properties of carbon tetrabromide. The length of time required for this reaction, and the very small relative amount of substance obtained, would, however, render this Published on 01 January 1871. Downloaded by Brown University 25/10/2014 10:39:25. quite inapplicable as a process for the preparation of the tetra- bromide. Action of Bromine on Carbon Disdphide in, presence of Certain Bromides. -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) . -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Used at Rocky Flats
. TASK 1 REPORT (Rl) IDENTIFICATION OF CHEMICALS AND RADIONUCLIDES USED AT ROCKY FLATS I PROJECT BACKGROUND ChemRisk is conducting a Rocky Flats Toxicologic Review and Dose Reconstruction study for The Colorado Department of Health. The two year study will be completed by the fall of 1992. The ChemRisk study is composed of twelve tasks that represent the first phase of an independent investigation of off-site health risks associated with the operation of the Rocky Flats nuclear weapons plant northwest of Denver. The first eight tasks address the collection of historic information on operations and releases and a detailed dose reconstruction analysis. Tasks 9 through 12 address the compilation of information and communication of the results of the study. Task 1 will involve the creation of an inventory of chemicals and radionuclides that have been present at Rocky Flats. Using this inventory, chemicals and radionuclides of concern will be selected under Task 2, based on such factors as the relative toxicity of the materials, quantities used, how the materials might have been released into the environment, and the likelihood for transport of the materials off-site. An historical activities profile of the plant will be constructed under Task 3. Tasks 4, 5, and 6 will address the identification of where in the facility activities took place, how much of the materials of concern were released to the environment, and where these materials went after the releases. Task 7 addresses historic land-use in the vicinity of the plant and the location of off-site populations potentially affected by releases from Rocky Flats. -
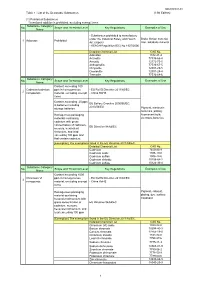
Table 1 : List of the Declarable Substances (1) Prohibited
DQ2000001-03 Table 1 : List of the Declarable Substances (13th Edition) (1) Prohibited Substances *Intentional addition is prohibited, excluding exempt items Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name - Substances prohibited to manufacture under the Industrial Safety and Health Brake friction material, 1 Asbestos Prohibited Act (Japan) filler, adiabatic material - REACH Regulation (EC) No 1907/2006 Detailed Chemical List CAS No. Asbestos 1332-21-4 Actinolite 77536-66-4 Amosite 12172-73-5 Anthophyllite 77536-67-5 Chrysotile 12001-29-5 Crocidolite 12001-28-4 Tremolite 77536-68-6 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 100 Cadmium/cadmium ppm in homogeneous - EU RoHS Directive 2011/65/EC 2 compounds material, excluding exempt - China RoHS items Content exceeding 20 ppm EU Battery Directive 2006/66/EC, in batteries including 2013/56/EU storage batteries Pigment, electronic materials, plating, Homogenous packaging fluorescent bulb, materials containing electrode,batteries cadmium with gross concentration of cadmium, EU Directive 94/62/EC mercury, hexavalent chromium, and lead exceeding 100 ppm and that contain cadmium [Exemption] The exemptions listed in the EU Directive 2011/65/EC. Detailed Chemical List CAS No. Cadmium 7440-43-9 Cadmium oxide 1306-19-0 Cadmium sulfide 1306-23-6 Cadmium chloride 10108-64-2 Cadmium sulfate 10124-36-4 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 1000 Chromium VI ppm in homogeneous - EU RoHS Directive 2011/65/EC 3 compounds material, excluding exempt - China RoHS items Homogenous packaging Pigment, catalyst, material containing plating, dye, surface hexavalent chromium with treatment gross concentration of EU Directive 94/62/EC cadmium, mercury, hexavalent chromium, and lead exceeding 100 ppm [Exemption] The exemptions listed in the EU Directive 2011/65/EC. -

DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS
criteria for a recommended standard . occupational exposure to DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE criteria for a recommended standard... OCCUPATIONAL EXPOSURE TO DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health September 1977 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DHEW (NIOSH) Publication No. 77-193 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and safety of workers exposed to an ever-increasing number of potential hazards at their workplace. The National Institute for Occupational Safety and Health has projected a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the health effects of exposure. The Secretary of Labor will weigh these recommendations along with other considerations such as feasibility and means of implementation in developing regulatory standards. It is intended to present successive reports as research and epidemiologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of the worker. I am pleased to acknowledge the contributions to this report on the decomposition products of fluorocarbon polymers by members of the NIOSH staff and the valuable constructive comments by the Review Consultants on the Decomposition Products of Fluorocarbon Polymers and by Robert B. -

Crystal Structure Transformations in Binary Halides
1 A UNITED STATES DEPARTMENT OF A111D3 074^50 IMMERCE JBLICAT10N NSRDS—NBS 41 HT°r /V\t Co^ NSRDS r #C£ DM* ' Crystal Structure Transformations in Binary Halides u.s. ARTMENT OF COMMERCE National Bureau of -QC*-| 100 US73 ho . 4 1^ 72. NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3—Laboratory Astrophysics 3—Cryo- 3 genics . -

Membrane Selection Guide
Zaiput Flow Technologies Membranes for Liquid-Liquid Separators Providing in-line liquid-liquid separation for flow chemistry Selection Guide A variety of membranes for your Zaiput separator are available to optimize separation performance and through- put. Membranes are available in both hydrophobic and hydrophilic, they are low cost and easy to replace. Membrane selection process The key parameters for identifying a suitable membrane for separation are the interfacial tension (IFT) between the two phases and the viscosity of the permeating phase (higher viscosities will decrease the throughput of the device). In general, the lower the interfacial tension, the smaller the pore size needs to be. However, smaller pore size reduces the maximum viscosity that can be accommodated by the membrane. A smaller pore size also lowers the total throughput of the device. For general applications, we recommend using a hydrophobic membrane and following the steps below: 1. Identify your mixture’s interfacial tension. Interfacial tension data for a variety of solvent systems is available at the end of this document, in literature or can be searched for online. For complex mixtures, an initial approxima- tion can be obtained by looking at the interfacial tension between the largest component of your system. Please note that salts give a modest increase to the interfacial tension and solvents miscible in both aqueous or organic will decrease it. Often a good estimate is enough, a more accurate value is helpful when dealing with low interfa- cial tension systems (<5 mN/m). 2. Know the viscosity of the permeating phase. Usually an estimate of the viscosity of your permeating phase (organic for a hydrophobic membrane) is enough. -

Isoflurane, 2-Halogenated Ethanols, and Halogenated Methanes Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Similar Mechanism
Isoflurane, 2-Halogenated Ethanols, and Halogenated Methanes Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Similar Mechanism. Authors: Joseph F. Cotten, M.D.,Ph.D1., Anita Luethy, M.D.1,2 1Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA, USA. 2Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland. Background: TASK-3 (KCNK9) tandem pore potassium channel proteins mediate a constitutive potassium conductance activated by several clinically relevant volatile anesthetics (e.g., halothane, isoflurane, sevoflurane, and desflurane); knockout mice lacking the TASK-3 potassium channel are resistant to the hypnotic and immoblizing effects of halothane and isoflurane. Purpose: To better understand the molecular mechanism by which TASK-3 channels are activated by anesthetics, we studied the functional concentration-response of wild-type TASK-3 potassium channels to isoflurane, to ethanol, and to several halogenated ethanols and methanes. We also studied the concentration-response of M159W TASK-3 to 2,2,2- trichloroethanol; the M159W TASK-3 mutant is known to be resistant to isoflurane activation (1). 2,2,2-trichloroethanol, notably, is an active metabolite of the sedative chloral hydrate; and 2,2,2-tribromoethanol is the active ingredient in Avertin, an injectable veterinary anesthetic. Methods: Wild-type and M159W TASK-3 function were studied by Ussing chamber voltage clamp analysis during transient expression in Fischer rat thyroid cell monolayers. Results: 2-halogenated ethanols activate wild-type TASK-3 with the following rank order for efficacy: 2,2,2-tribromo > 2,2,2-trichloro > chloral hydrate > 2,2-dichloro > 2-chloro ≈ 2,2,2- trifluoro > ethanol (Table 1).