19700018737.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS
criteria for a recommended standard . occupational exposure to DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE criteria for a recommended standard... OCCUPATIONAL EXPOSURE TO DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health September 1977 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DHEW (NIOSH) Publication No. 77-193 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and safety of workers exposed to an ever-increasing number of potential hazards at their workplace. The National Institute for Occupational Safety and Health has projected a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the health effects of exposure. The Secretary of Labor will weigh these recommendations along with other considerations such as feasibility and means of implementation in developing regulatory standards. It is intended to present successive reports as research and epidemiologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of the worker. I am pleased to acknowledge the contributions to this report on the decomposition products of fluorocarbon polymers by members of the NIOSH staff and the valuable constructive comments by the Review Consultants on the Decomposition Products of Fluorocarbon Polymers and by Robert B. -

CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry 3M MN04855110
CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry BY H. G. BRYCE f~Iinnesota ~Vlining and Manufacturing Company, St. Paul, Minnesota I. Introduction ....................................................... 297 II. Historical and Economic Factors ..................................... 298 III. Characteristic Properties of Fluorocarbons .............................. 302 A. Bond Energies and Bond Distances ............................... 302 B. Boiling Points and Melting Points ................................ 303 C. Surface Energy ................................................ 304 D. Stability ...................................................... 309 E. Electrical Properties ............................................ 312 F. Polarity of Oxides or Nitrides and Effect of Structure ............... 312 G. Solubility ..................................................... 314 IV. Refrigerants and Propellants ......................................... 316 A. Properties ..................................................... 317 B. Refrigeration Characteristics ..................................... 320 C. Applications for Specific Compounds ............................. 323 V. Heat Transfer Media ................................................ 323 A. Heat Transfer Processes ........................................ 323 B. Miniaturization ................................................ 324 C. Application of FIuorocarbon Fluids to Heat Transfer Problems ....... 325 D. Properties of Fluorocarbon Fluids ............................... -
C1-C4 Halogenated HC Info 16FEB2018.Xlsx
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) Feb 28, 2018 Administrative Council Mtg - Printed 2/16/20184:11 PM Reported Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR, mfr literature) TURA TSCA CDR Chemical CAS/Chemica chemical name chemical fire 2015 Chemical Name Pesti- blowing feedstock / supressant / propell- Inventory? TRI? List l Number (synonyms) formula Solvent refriger-ant etchant Other Tier II cide agent intermed-iate flame ant status* retardant insulation for refrigerators, freezers, commercial refrigeration equipment, refrigerated containers and LNG ships; spray foam insulation; insulated metal panels; slabstock and molded flexible foam; refrigerant for chillers; and solvents for metal cleaning and electronics, and circuit flush. https://www.honeywell-blowingagents.com/?document=solstice-lba-technical- HFO-1233zd (E) brochure&download=1 Hydro-fluoro-olefin C H ClF YYY yhttps://www.honeywell-refrigerants.com/americas/product/solstice-zd/ (HFO) 3 2 3 CDR 2016 Honeywell: 100% as propellants and blowing agents for plastics product mfg; R-1233zd CDR Honeywell Solstice® ZD refrigerant, Solstice® 1233zdE, Solstice Blowing Agent; Arkema (current Forane® 1233zd blowing agent for spray PU foam, appliance insulation, etc. and solvent (may Honeywell not be available in US) product) C1-C4 Cat 102687-65-0 1-Chloro-3,3,3-trifluoropropene Y 106‐95‐6 Allyl bromide 2-Propenyl bromide C3H5Br Y y Manufacture of synthetic perfumes, other allyl compounds; Insecticidal fumigant; 1-Propene, 3-bromo- Chemical intermediate in organic synthesis, for resins (copolymer with sulfur dioxide) and fragrances; As a fumigant (if quite volatile) or as a contact poison; C1-C4 Cat Used in the manufacture of plastics and dyestuff. -
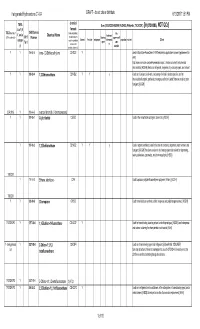
List for Comments C1-C4 Halogenated Hydrocarbons
Halogenated Hydrocarbons C1-C4 DRAFT - do not cite or distribute 6/13/201711:51 PM chemical TURA Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR ) [In process, NOT QCd] List? (If formula TSCA Inventory? CAS/Chemica (Yellow highlighting = fire Y, not in Chemical Name feedstock/ (Y/N - student ck) 2015 l Number multiple halogens blowing supressant/fl Solvent Pesticide refrigerant intermedia propellant etchant Other categor could be substituted agent ame Tier II to create other te y) retardant possible substances) Y Y 156-60-5 trans-1,2-Dichloroethylene C2H2Cl2 Y used in MicroCare PowerClean II PW2 electronics applications solvent (replacement for nPB) http://www.microcare.com/p-64-powerclean.aspx; Used as a solvent and chemical intermediate; [ACGIH] Used as a refrigerant, degreaser, dry cleaning agent, and solvent YY 106-93-4 1,2-Dibromoethane C2H4Br2 Y Y y Usedfor perfumes, as a fumigant, adhesives, a solvent, lacquers, a scavenger oils, and forresins; lead [HSDB]in leaded gasoline, and an intermediate in organic synthesis; no longer used in the United States as a soil or grain fumigant; [ACGIH] CDR 2015 Y 106-94-5 n-propyl bromide (1-bromopropane) YY 107-05-1 Allyl chloride C3H5Cl Used in the manufacture of organic chemicals; [ACGIH] Y 107-06-2 1,2-Dichloroethane C2H4Cl2 Y Y y Used in organic synthesis; used in the past as a solvent, degreaser, paint remover, and fumigant; [ACGIH] Has been used as a dry cleaning agent and solvent for degreasing, resins, adhesives, coasmetics, and pharmaceuticals; [HSDB] TRI/CDR Y 116-14-3 Ethene, tetrafluoro- -

Vinyl Fluoride
FINAL Report on Carcinogens Background Document for Vinyl Fluoride Meeting of the NTP Board of Scientific Counselors Report on Carcinogens Subcommittee Prepared for the: U.S. Department of Health and Human Services Public Health Services National Toxicology Program Research Triangle Park, NC 27709 Prepared by: Technology Planning and Management Corporation Canterbury Hall, Suite 310 4815 Emperor Blvd Durham, NC 27703 Contract Number NOI-ES-85421 RoC Background Document for Vinyl Fluoride Criteria for Listing Agents, Substances or Mixtures in the Report on Carcinogens US Department of Health and Human Services National Toxicology Program Known to be Human Carcinogens: There is sufficient evidence of carcinogenicity from studies in humans which indicates a causal relationship between exposure to the agent, substance or mixture and human cancer. Reasonably Anticipated to be Human Carcinogens: There is limited evidence of carcinogenicity from studies in humans which indicates that causal interpretation is credible but that alternative explanations such as chance, bias or confounding factors could not adequately be excluded; or There is sufficient evidence of carcinogenicity from studies in experimental animals which indicates there is an increased incidence of malignant and/or a combination of malignant and benign tumors: (1) in multiple species, or at multiple tissue sites, or (2) by multiple routes of exposure, or (3) to an unusual degree with regard to incidence, site or type of tumor or age at onset; or There is less than sufficient evidence of carcinogenicity in humans or laboratory animals, however; the agent, substance or mixture belongs to a well defined, structurally-related class of substances whose members are listed in a previous Report on Carcinogens as either a known to be human carcinogen, or reasonably anticipated to be human carcinogen or there is convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans. -

Guide to the Safe Handling of Fluoropolymer Resins
1 Consult the following information in fire emergencies and other emergencies. Be sure to review the details of proper handling and use of fluoropolymers in this Guide. Questions about proper use of fluoropolymer resins should be directed to the resin supplier. Questions about this Guide or the Fluoropolymers Division (FPD) should be directed to The Plastics Industry Association IN CASE OF FIRE • Fluoropolymers do not burn without an external source of heat (See page 40.) • The principle decomposition products in a fire are hydrogen fluoride (HF), carbonyl fluoride (COF2), and carbon monoxide (CO) and low molecular weight fluoropolymers. (See page 15.) • Wear self-contained breathing apparatus (SCBA) to protect skin and eyes from contact with HF (See page 35.) • Wear full turnout gear or Level A equipment, to protect skin, eyes and respiratory system from contact with HF (See page 35) • Decontaminate personnel and equipment with water wash-down after fire and smoke exposure, as well as after salvage and overhaul. (See page 36.) • See the National Fire Protection Association’s publication, Fire Protection Guide to Hazardous Materials, 13th Edition and 29 CFR 1910.10 (k), “Hazardous waste operations and emergency response,” for guidelines. EMERGENCY RESPONSE o • Hazardous fumes from PTFE may be produced at temperatures above 625 F o (330 C). (See pages 14 and 19.) o o • Toxic fumes from PTFE may be formed at 840 F (450 C) and above. (See pages 14 and 19.) • Hazardous vapor and fumes will be liberated during a fire. (See page 40.) EMERGENCY RESPONSE NUMBERS (see page 43 for additional information): • Transportation Emergency for AGC Chemicals Americas, Arkema, Daikin America, CHEMOURS, and Solvay Solexis: (CHEMTREC) – (800) 424-9300 • For Dyneon/3M ONLY: (800) 364-3577 2 Table of Contents NOTE TO USERS .......................................................................................................... -

(PFAS) a Catena Lunga Di Minore Impatto Ambientale E Sanitario
ISTITUTO DI RICERCHE FARMACOLOGICHE MARIO NEGRI Via Giuseppe La Masa, 19 - 20156 Milano MI - Italy - www.marionegri.it tel +39 02 39014.1 - fax +39 02 354.6277 - [email protected] Studio finalizzato all’individuazione di potenziali sostituti delle sostanze perfluoroalchiliche (PFAS) a catena lunga di minore impatto ambientale e sanitario Emilio Benfenati, Edoardo Carnesecchi, Caterina Leone, Elia Bertuzzi, Anna Lombardo, Alessandra Roncaglioni Istituto di Ricerche Farmacologiche Mario Negri- IRCCS Laboratorio di Chimica e Tossicologia dell’Ambiente IRCCS - D.M. 18 gennaio 2013 Gazzetta Uff. n. 34 del 09/02/2013 - confermato ai sensi del D.M. 8 maggio 2018 Gazzetta Uff. n. 144 del 23/06/2018I CONTRIBUTI PER LA RICERCA VERSATI ALL'ISTITUTO SONO FISCALMENTE DEDUCIBILI DAL REDDITO (Gazzetta Uff. N. 268 del 16/11/2016 e Gazzetta Uff. N. 290 del 13/12/2016 ) FONDAZIONE PER RICERCHE ERETTA IN ENTE MORALE, D.P.R. 361 DEL 5/4/1961 - REGISTRO PERSONE GIURIDICHE PREFETTURA MILANO N.227CONTO CORRENTE POST. N.58337205 - COD. FISC. E PARTITA IVA 03254210150 - ANAGRAFE NAZIONALE RICERCHE COD.G1690099 Istituto con sistema di gestione qualità UNI EN ISO 9001:2015 certificato da Certiquality (Il dettaglio delle attività oggetto del certificato N. 6121 è disponibile sul sito http://www.marionegri.it/mn/it/sezioni/formazione/index.html) Indice Background .................................................................................................................................. 3 Summary ..................................................................................................................................... -

Exergetic Life Cycle Assessment of Electrospun Polyvinylidene Fluoride Nanofibers Salman Ali Abbasi University of South Florida, [email protected]
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 10-29-2014 Exergetic Life Cycle Assessment of Electrospun Polyvinylidene Fluoride Nanofibers Salman Ali Abbasi University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Mechanical Engineering Commons Scholar Commons Citation Abbasi, Salman Ali, "Exergetic Life Cycle Assessment of Electrospun Polyvinylidene Fluoride Nanofibers" (2014). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/5346 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Exergetic Life Cycle Assessment of Electrospun Polyvinylidene Fluoride Nanofibers by Salman Ali Abbasi A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Mechanical Engineering Department of Mechanical Engineering College of Engineering University of South Florida Major Professor: Delcie Durham, Ph.D. Sylvia Thomas, Ph.D. Alex A. Volinsky, Ph.D. Date of Approval: October 29, 2014 Keywords: LCA, Sustainability, Exergy, Nanotechnology, Hybrid Copyright © 2014, Salman Ali Abbasi DEDICATION To Rafia. ACKNOWLEDGMENTS I wish to express my deep gratitude to Dr. Delcie Durham, my adviser, for her precious advice and guidance throughout the course of completion of this thesis, and for bearing with me through those long office meetings. I thank Brian Bell for his help in experimentation and for sharing his valuable ideas. I have learnt some of the key concepts of experimental design and critical thinking from him, and I am grateful for that.