Highlights of This Issue Identity Protection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Changing Lives: Engaging People, Places and Systems to Improve Health Outcomes, 2014
Changing Lives Engaging People, Places and Systems to Improve Health Outcomes 2014 Highmark Foundation Giving Report www.highmarkfoundation.org The image on the cover of this report is meant to represent positive change and improvement, and speaks directly to the positive impact the Foundation has on the people and communities it serves. 1 Changing Lives: Engaging People, Places and Systems to Improve Health Outcomes Mission The Highmark Foundation is a private, charitable organization of Highmark Inc. that supports initiatives and programs aimed at improving community health. The Foundation’s mission is to improve the health, well-being and quality of life for individuals who reside in the communities served by Highmark Inc. The Foundation strives to support evidence-based programs that impact multiple counties and work collaboratively to leverage additional funding to achieve replicable models. For more information, visit www.highmarkfoundation.org. Contents Board Members and Officers 3 Introduction to the Highmark Foundation 4 Highmark Foundation Grants 8 Highmark Foundation in the News 18 2014 Highmark Foundation Giving Report 1 The Highmark Foundation was established in 2000 to improve The initiatives funded by the Foundation fall within four the health and well-being of people living in the diverse categories: chronic disease, family health, service delivery communities served by Highmark Inc. We do this by awarding systems and healthy communities. These are the areas where high-impact grants to charitable organizations, hospitals and we have seen the greatest needs and remain our primary schools that develop programs to advance community health. areas of focus. The Foundation’s greatest successes are strong partnerships As we look ahead to 2015 and beyond, the Foundation with regional, national and global organizations with similar remains committed to improving the health and well-being missions, working to raise awareness of community health of communities throughout Pennsylvania and West Virginia. -

2002 Opinions
ERIE COUNTY LEGAL JOURNAL (Published by the Committee on Publications of the Erie County Legal Journal and the Erie County Bar Association) Reports of Cases Decided in the Several Courts of Erie County for the Year 2002 LXXXV ERIE, PA JUDGES of the Courts of Erie County during the period covered by this volume of reports COURTS OF COMMON PLEAS HONORABLE WILLIAM R. CUNNINGHAM -------- President Judge HONORABLE GEORGE LEVIN ---------------------------- Senior Judge HONORABLE ROGER M. FISCHER ----------------------- Senior Judge HONORABLE FRED P. ANTHONY --------------------------------- Judge HONORABLE SHAD A. CONNELLY ------------------------------- Judge HONORABLE JOHN A. BOZZA ------------------------------------ Judge HONORABLE STEPHANIE DOMITROVICH --------------------- Judge HONORABLE ERNEST J. DISANTIS, JR. ------------------------- Judge HONORABLE MICHAEL E. DUNLAVEY -------------------------- Judge HONORABLE ELIZABETH K. KELLY ----------------------------- Judge HONORABLE JOHN J. TRUCILLA --------------------------------- Judge Volume 85 TABLE OF CASES -A- Ager, et al. v. Steris Corporation ------------------------------------------------ 54 Alessi, et al. v. Millcreek Township Zoning Hearing Bd. and Sheetz, et al. 77 Altadonna; Commonwealth v. --------------------------------------------------- 90 American Manufacturers Mutual Insurance Co.; Odom v. ----------------- 232 Azzarello; Washam v. ------------------------------------------------------------ 181 -B- Beaton, et. al.; Brown v. ------------------------------------------------------------ -

Pennsylvania's Largest Employers (At Least 1,000 Employees)
Pennsylvania's Largest Employers (At Least 1,000 Employees) 1st Quarter, 2018 Combined Government Ownerships Center for Workforce Information & Analysis (877) 4WF-DATA • www.workstats.dli.pa.gov • [email protected] September 2018 Rank Employer Rank Employer 1 Federal Government 51 ACME Markets Inc 2 State Government 52 Aerotek Inc 3 Wal-Mart Associates Inc 53 Geisinger Medical Center 4 Trustees of the University of PA 54 Reading Hospital 5 City of Philadelphia 55 Dolgencorp LLC 6 Pennsylvania State University 56 Carnegie Mellon University 7 Giant Food Stores LLC 57 Abington Memorial Hospital 8 School District of Philadelphia 58 FedEx Ground Package System Inc 9 UPMC Presbyterian Shadyside 59 Highmark Inc 10 United Parcel Service Inc 60 Kohl's Department Stores Inc 11 PNC Bank NA 61 Rite Aid of Pennsylvania Inc 12 University of Pittsburgh 62 Marmaxx Operating Corporation 13 Lowe's Home Centers LLC 63 The Hershey Company 14 Weis Markets Inc 64 Wells Fargo NA 15 The Children's Hospital of Philadelphia 65 Temple University Hospital Inc 16 Comcast Cablevision Corp (PA) 66 York Hospital 17 Home Depot USA Inc 67 SmithKline Beecham Corporation 18 PA State System of Higher Education 68 Starbucks Corporation 19 Giant Eagle Inc 69 Boscov's Department Store LLC 20 Amazon.com DEDC LLC 70 School District of Pittsburgh 21 The Vanguard Group Inc 71 UPMC Pinnacle Hospitals 22 Target Corporation 72 Geisinger Clinic 23 Merck Sharp & Dohme Corporation 73 Dick's Sporting Goods Inc 24 Western Penn Allegheny Health 74 Hershey Entertainment & Resorts Co 25 -
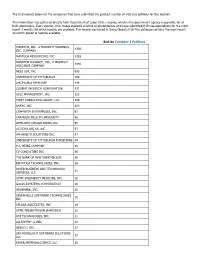
The H1B Records Below List the Companies That Have Submitted the Greatest Number of H1B Visa Petitions for This Location
The H1B records below list the companies that have submitted the greatest number of H1B visa petitions for this location. This information was gathered directly from Department of Labor (DOL) records, which is the government agency responsible for all H1B submissions. Every quarter, DOL makes available a listing of all companies who have submitted H1B visa applications for the most recent 3 months for which records are available. The records contained in Going Global's H1B Plus database contains the most recent 12-month period of records available. Sort by Company | Petitions MASTECH, INC., A MASTECH HOLDINGS, 4339 INC. COMPANY MASTECH RESOURCING, INC. 1393 MASTECH ALLIANCE, INC., A MASTECH 1040 HOLDINGS COMPANY NESS USA, INC. 693 UNIVERSITY OF PITTSBURGH 169 UHCP D/B/A UPMC MEP 144 COGENT INFOTECH CORPORATION 131 SDLC MANAGEMENT, INC. 123 FIRST CONSULTING GROUP, LLC 104 ANSYS, INC. 100 COMPUTER ENTERPRISES, INC. 97 CARNEGIE MELLON UNIVERSITY 96 INTELLECT DESIGN ARENA INC. 95 ACCION LABS US, INC. 63 HM HEALTH SOLUTIONS INC. 57 UNIVERSITY OF PITTSBURGH PHYSICIANS 44 H.J. HEINZ COMPANY 40 CV CONSULTING INC 36 THE BANK OF NEW YORK MELLON 36 INFOYUGA TECHNOLOGIES, INC. 36 BAYER BUSINESS AND TECHNOLOGY 31 SERVICES, LLC UPMC EMERGENCY MEDICINE, INC. 28 GALAX-ESYSTEMS CORPORATION 26 HIGHMARK, INC. 25 SEVEN HILLS SOFTWARE TECHNOLOGIES 25 INC VELAGA ASSOCIATES, INC 24 UPMC PRESBYTERIAN SHADYSIDE 22 DVI TECHNOLOGES, INC. 21 ALLEGHENY CLINIC 20 GENCO I. INC. 17 SRI MOONLIGHT SOFTWARE SOLUTIONS 17 LLC BAYER MATERIALSCIENCE, LLC 16 BAYER HEALTHCARE PHARMACEUTICALS, 16 INC. VISVERO, INC. 16 CYBYTE, INC. 15 BOMBARDIER TRANSPORTATION 15 (HOLDINGS) USA, INC. -

Download Our 2020 Corporate Profile
HIGHMARK HEALTH 2020 CORPORATE PROFILE 1 Living Health: Together for a Purpose 2020 Corporate Profile HIGHMARK HEALTH 2020 CORPORATE PROFILE 2 Our vision is a world Highmark Health is committed to reinventing the health experience so that everything and everyone works together for the health of those we serve. Boldly challenging unsustainable health care models of the past, we are where everyone building a new model, Living Health, to simplify the customer experience, free up clinicians to focus on care, embraces health. and leverage innovative technology and partnerships to deliver truly individualized health planning. We believe that creating a remarkable health experience will drive better health outcomes and lower total cost of care. Like our work, our financial priorities also serve our social purpose of building stronger communities of Our mission is to healthier people. In 2020, that included more than $750 million invested to support customers, providers, and create a remarkable communities during the pandemic, and another $760 million in capital investments. health experience, A DIVERSE PORTFOLIO OF LEADING HEALTH COMPANIES freeing people to Highmark Health’s diverse portfolio of aliates and subsidiaries meets a broad spectrum of health needs for be their best. consumers, business customers, and government entities. Highmark Inc. and its Blue-branded aliates HM Health Solutions combines technology and (Highmark Health Plans) proudly cover the leading industry knowledge to deliver business insurance needs of millions of individuals, families, solutions to health plan payers so they can run their and seniors, while also oering a variety of health- organizations eciently in a competitive and ever- related products and services. -

2019 Highmark Health Annual Report: Corporate Profile
HIGHMARK HEALTH 2019 CORPORATE PROFILE 1 Embracing Health in Remarkable Ways GLORIA, AHN PATIENT ▶ 2019 Corporate Profile HIGHMARK HEALTH 2019 CORPORATE PROFILE 2 Our vision is a world Highmark Health is committed to reinventing the health experience so that everything and everyone works together seamlessly for the health of those we serve. Boldly challenging unsustainable health care models of where everyone the past, we are simplifying the customer experience, freeing up clinicians to focus on care, and leveraging embraces health. innovative technology and partnerships to deliver truly individualized health planning. We believe that creating a remarkable health experience is also the path to better health outcomes and lower total cost of care. Like our work, our organizational giving — totaling $206.5 million in 2019 — centers on our larger social purpose of Our mission is to building stronger communities of healthier people. create a remarkable A DIVERSE PORTFOLIO OF LEADING HEALTH CARE COMPANIES health experience, Highmark Health’s strong and diverse portfolio of businesses meets a broad spectrum of health needs for freeing people to consumers, business customers, and government entities. be their best. Highmark Inc. and its Blue-branded affiliates (Health HM Home & Community Services specializes Plan) proudly cover the insurance needs of millions of in population health management solutions that individuals, families and seniors, while also offering a benefit payers, providers, and customers. variety of health-related products and services. HM Insurance Group works to protect businesses Allegheny Health Network (AHN) has more and their employees from the financial risks than 250 clinical facilities, including hospitals associated with catastrophic health care costs. -

Highmark Inc. Company Overview
Highmark Inc. Company Overview Vision A world where everyone embraces health Mission To create a remarkable health experience, freeing people to be their best Key Statistics • Founded Mid 1930s • Number of employees 6,000 • 5.6 million members are covered by Highmark Inc. • Highmark Health had $18 Billion in Operating Revenue in 2019 • Highmark Inc., together with its Blue-branded affiliates, collectively comprise the fourth-largest overall Blue Cross Blue Shield-affiliated organization in the country based on membership. Company Headquarters • Pittsburgh, Pa. Community Support • In 2019, the Health Plans' corporate giving benefited hundreds of organizations, donating more than $17 million in the areas of western, central, and northeastern Pennsylvania, West Virginia, and Delaware. In addition, Highmark's signature program, Walk for a Healthy Community, along with Adopt-a-School programs initiated by employees in Pittsburgh and Erie, and National Make a Difference Day of volunteering, provided significant benefits to individuals and the communities the Health Plans proudly serve. 1 Products • Highmark Inc. is a national, diversified health care partner based in Pittsburgh that serves members across the United States through its businesses in health insurance, dental insurance, and reinsurance. We offer a variety of plans in our core service areas. • Our diversified businesses also provide a broad spectrum of specialty products, such as dental insurance, and reinsurance to 50 million Americans across all 50 states and the District of Columbia. • Our diversified businesses rank among the nation's leaders in their categories: o Fifth largest dental insurance carrier in the U.S. o Among the top five largest stop loss carriers Awards • Medicare Advantage plans recognized for member satisfaction For the fourth consecutive year, Highmark's Medicare Advantage plans received high rankings in the annual J.D. -

Fifth Annual Father's Day Pledge Media Advisory
MEDIA ADVISORY Contact: Maggie Stasko, 412-642-7700 [email protected] Pittsburgh Says “NO MORE” to Domestic Violence and Sexual Assault 5th Annual Father’s Day Pledge Ceremony will take place at U.S. Steel Tower Plaza on June 13 – largest gathering to date expected with participation by several corporations WHAT: Father’s Day Pledge Public Signing Ceremony to End Gender Violence On Thursday, June 13, prominent business, civic and community leaders, and public officials will lend their voices to the prevention of domestic violence and sexual assault at the 5th annual Father’s Day Pledge Ceremony in downtown Pittsburgh. This year’s public gathering is expected to be the largest yet with groups from UPMC, Koppers, Ernst & Young LLC, PNC, KPMG, Highmark Health and several other organizations participating. During the rally, the leaders will recite and sign the pledge to end gender-based violence. The program will conclude with a public pledge signing. The event is hosted by Southwest PA Says No More, an initiative supported by FISA Foundation, The Heinz Endowments and United Way of Southwestern Pennsylvania. WHEN: Thursday, June 13, 2019, 12:00 p.m. to 1:00 p.m. WHERE: U.S. Steel Tower Plaza, 600 Grant Street, Downtown Pittsburgh (If it rains, the event will be held in the upper lobby of U.S. Steel Tower.) AGENDA: 12:00 p.m. Welcome by emcee Sally Wiggin 12:05 p.m. Opening remarks Kristy Trautmann, FISA Foundation Adam Baron, United Way of Southwestern PA 12:12 p.m. Prevention takes leadership The Honorable Rich Fitzgerald, County Executive The Honorable William Peduto, Mayor, City of Pittsburgh 12:20 p.m. -

Master of Public Management Part-Time Courses Big-Time Career Impact
MASTER OF PUBLIC MANAGEMENT PART-TIME COURSES BIG-TIME CAREER IMPACT Heinz College’s Master of Public Management (MPM) program combines our unique formula of analytics, technology, and leadership practice that you would find in any of our full-time management programs. IS MPM RIGHT FOR ME? #1 · All classes meet once a week, in the evening · Heinz College is #1 in Analytics Education Heinz College #1 in · Over 90% of students receive scholarship Analytics Education · MPM cohorts are small, yet diverse - INFORMS UPS George D. Smith Prize 2016 · 2 years to complete, part-time · No GRE or GMAT required MPM is designed with working professionals in mind. Your MPM cohort will be composed of fellow decision-makers from influential sectors, such as health care, education, finance, government, and non-profits. Accelerate your current career path or pivot to a new focus or field. You will graduate from Heinz College with an analytical skillset that will set you apart in any organization. See the reverse for more details about our curriculum, and you’ll see what we mean. heinz.cmu.edu MPM Curriculum Core Coursework provides depth in Elective Coursework allows you to tailor Analytics, Technology, Communication, the degree to your interest areas. Current and Management, including: elective offerings include classes in: · Business Analytics · Analytics/Technology · Optimization & Risk Modeling · Arts Management · Organizational Management · Cybersecurity · Database Management · Design for Managers · Financial Analysis · Economic Development & Planning · -

Highmark Health 2013 Annual Report
Leading the transformation of Health Care 2013 Annual Report Leading the transformation of Health Care As a Pittsburgh-based health and wellness enterprise, Highmark Health serves 35 million individuals through our affiliates. We maintain a steadfast commitment to making high-quality health care accessible, understandable and affordable. This report details how Highmark Health and our affiliates work every day to positively impact the lives of the people we serve. Letter From the Chairman of the Board Highmark Health and its affiliated companies, Highmark Inc. and the Allegheny Health Network, had a particularly strong year in 2013. We achieved significant success and continued to transform the company through diversification and new approaches for managing costs and improving quality and the customer experience. Our strong performance provides us with a solid foundation to meet the opportunities and challenges we will face in 2014. Creating an integrated health care delivery system We are charting a new course in health care that is focused on integration, innovation and meeting the needs of the consumer. In 2013, we took a bold step consistent with our strategy for growth and created the Allegheny Health Network (AHN) through affiliations with Jefferson Hospital, Saint Vincent Health System and the West Penn Allegheny Health System. This offers us new opportunities to work on behalf of our customers and members to implement an entirely new health care delivery model and better align payments with patient care. These hospitals are important community assets, and we intend to apply their clinical expertise to ensure that residents of Western Pennsylvania get the best health care possible. -

Weekly Capitol Hill Report July 31, 2020
Issues for the week ending July 31, 2020 In this Issue: Federal Issues Legislative Federal Issues Legislative House Postpones Recess as COVID-19 Relief House Postpones Recess as COVID-19 Talks Stall Relieve Talks Stall U.S. House Majority Leader Steny Hoyer (D-MD) Coalition Urges Congressional Leaders to End Surprise Billing announced Friday that the chamber would remain in session and forgo its traditional August recess until Congress finishes its next coronavirus bill. The Regulatory announcement came after Senate Republicans CMS Releases Part D National Average released a series of bills collectively known as the Monthly Bid Amount and Other Bid/Payment- HEALS Act to address the crisis last week, sparking Related Information negotiations with Democrats who ushered the Appeals Court Upholds Payment Cut for HEROES Act through the House in May. 340B Hospitals CMS Seeks Input for Rule to Mandate E- Despite talks between both parties and the White prescribing of Controlled Substances House, the sides remain $2 trillion apart and State Medicaid Directors Urge CMS to Suspend Medicaid Fiscal Accountability Rule negotiators reportedly made little ground last week Federal COVID-19 Policy Guidance and Other despite the fact that millions remain unemployed and Developments enhanced unemployment benefits expire July 31. Federal Court Issues Temporary Nationwide Stay of “Public Charge” Rule What’s next: It seems unlikely a resolution will be Fifth Circuit Decision in Favor of Health Plans reached soon, although President Trump has Medicaid MCO Health Insurance Tax Lawsuit expressed a willingness to cede ground to the Democrats to get a deal. Key points of contention are levels of funding for state and local governments, State Issues liability protections for reopening facilities, the amount Delaware of additional unemployment benefits, and additional Regulatory COVID-19 testing dollars. -
ACC-Pittsburgh-Newsletter.Pdf
Inside 4Q2013 2 ....Taking Charge of Legal Spending - The ACC Value Challenge 4 ....Internal Investigations from a Practical Perspective 5 .....ACC News 6 ....Past Event Photos 8 .....Five Tips for Preparing Effective Intellectual Property Assignments 9 ....Upcoming Events 10 ...Welcome New Members! 10 ..Board Members and Contacts FOCUS President’s Message Kevin Whyte, Carmeuse Lime & Stone My term as President, which I have • We arranged a joint CLE/ Where have we fallen short? enjoyed immensely, has come to a close. networking event with the One area in which we !e ACC is a wonderful organization that Washington County Bar have not made progress is o"ers many resources and opportuni- Association. advocacy for certain posi- ties to in- house lawyers. I intend to stay • We established the use of tions in which the in-house involved and to take advantage of such LinkedIn to communicate bar has a special interest. resources and opportunities. with our membership. We ACC National is very active First, I would like to thank Barb Dudek, would hope that the use with respect to advocacy our Chapter Administrator. Barb’s experi- of this tool will increase as initiatives. I have not done a ence, attention to detail, dedication and members become increas- good job of supporting such creativity ensure that things get done ingly aware of the ease of e"orts. Certainly, this is an promptly and in a professional man- communicating by this medium. area in which we can improve. ner. Second, I would like to thank all of • We initiated a program to reward an As mentioned in an earlier Focus article, our o#cers and board members.