Weekly Capitol Hill Report July 31, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SENATE—Wednesday, October 26, 2005
October 26, 2005 CONGRESSIONAL RECORD—SENATE 23679 SENATE—Wednesday, October 26, 2005 The Senate met at 9:30 a.m. and was pose of these amendments, and we will tion drug program that is about to called to order by the President pro announce when Senators can expect take effect. This flaw is a ticking time tempore (Mr. STEVENS). those votes. bomb for more than 6 million Ameri- I remind my colleagues that a clo- cans, for our communities and our PRAYER ture motion was filed last night on the health care providers. That fuse is The Chaplain, Dr. Barry C. Black, of- Labor-HHS appropriations bill. That going to detonate on January 1. fered the following prayer: cloture vote will occur on Thursday We cannot allow low-income seniors Let us pray. morning. Under rule XXII, Senators and the disabled to lose their direct O God our rock, exalted above all have until 1 o’clock today to file their coverage. We cannot leave our doctors blessings and praise, the host of Heav- first-degree amendments at the desk. and hospitals and nursing homes un- en worships You. Today we praise You We will finish this bill this week. It is prepared for the biggest change in dec- for the opportunity of serving our up to the Senate to decide if we are ades. And we should not be pushing country in the Senate. Incline our going to be here late Thursday or Fri- hundreds of thousands of people who hearts to do Your will and set a guard day, but we will finish the bill. -

Changing Lives: Engaging People, Places and Systems to Improve Health Outcomes, 2014
Changing Lives Engaging People, Places and Systems to Improve Health Outcomes 2014 Highmark Foundation Giving Report www.highmarkfoundation.org The image on the cover of this report is meant to represent positive change and improvement, and speaks directly to the positive impact the Foundation has on the people and communities it serves. 1 Changing Lives: Engaging People, Places and Systems to Improve Health Outcomes Mission The Highmark Foundation is a private, charitable organization of Highmark Inc. that supports initiatives and programs aimed at improving community health. The Foundation’s mission is to improve the health, well-being and quality of life for individuals who reside in the communities served by Highmark Inc. The Foundation strives to support evidence-based programs that impact multiple counties and work collaboratively to leverage additional funding to achieve replicable models. For more information, visit www.highmarkfoundation.org. Contents Board Members and Officers 3 Introduction to the Highmark Foundation 4 Highmark Foundation Grants 8 Highmark Foundation in the News 18 2014 Highmark Foundation Giving Report 1 The Highmark Foundation was established in 2000 to improve The initiatives funded by the Foundation fall within four the health and well-being of people living in the diverse categories: chronic disease, family health, service delivery communities served by Highmark Inc. We do this by awarding systems and healthy communities. These are the areas where high-impact grants to charitable organizations, hospitals and we have seen the greatest needs and remain our primary schools that develop programs to advance community health. areas of focus. The Foundation’s greatest successes are strong partnerships As we look ahead to 2015 and beyond, the Foundation with regional, national and global organizations with similar remains committed to improving the health and well-being missions, working to raise awareness of community health of communities throughout Pennsylvania and West Virginia. -

ASE Lobbyist Confernce Federal Update Chris Rogers September
ASE Lobbyist Conference Federal Update Chris Rogers November 2020 Overview ■ Biden Transition (5-step plan to reopen schools) □ Controlling the Virus □ National Safety Guidelines □ Emergency Funding for Public Schools □ High-Quality Learning during the COVID-19 Pandemic □ COVID-19 Educational Equity Gap ■ Biden K-12 Education Proposals & Outlook ■ Lame Duck Session □ FY 2021 Appropriations □ COVID 5 2 Controlling the Virus Implement nationwide testing-and-tracing, including doubling the number of drive- Implement through testing sites; Establish a sustainable supply chain for PPE, including fully utilizing the Defense Establish Production Act to ensure enough masks are for every school in America every day; Protect Protect older Americans and others at high-risk populations; Provide Provide schools and small businesses with the resources they need to reopen safely. 3 National Safety Guidelines ■ Biden agrees with AASA, schools in areas with high levels of COVID-19 community spread should not be compelled to reopen against the judgement of local experts. Currently, current lack of clarity is paralyzing for schools. ■ Biden plans to support the efforts of Local Education Agencies by issuing federal reopening guidelines that answer basic questions many school systems have. 1. How low does the community infection rate need to be to reopen and at what point should schools shut down again if cases rise? 2. What are safe maximum class sizes? 3. 4If schools cannot accommodate everyone, who should return to the classroom first? Emergency Funding for Public Schools Biden’s 3rd step is to provide additional funding to public schools to contend with the coronavirus outbreak. Thus far, Biden has requested the following aid to support LEA’s reopening strategies. -

2002 Opinions
ERIE COUNTY LEGAL JOURNAL (Published by the Committee on Publications of the Erie County Legal Journal and the Erie County Bar Association) Reports of Cases Decided in the Several Courts of Erie County for the Year 2002 LXXXV ERIE, PA JUDGES of the Courts of Erie County during the period covered by this volume of reports COURTS OF COMMON PLEAS HONORABLE WILLIAM R. CUNNINGHAM -------- President Judge HONORABLE GEORGE LEVIN ---------------------------- Senior Judge HONORABLE ROGER M. FISCHER ----------------------- Senior Judge HONORABLE FRED P. ANTHONY --------------------------------- Judge HONORABLE SHAD A. CONNELLY ------------------------------- Judge HONORABLE JOHN A. BOZZA ------------------------------------ Judge HONORABLE STEPHANIE DOMITROVICH --------------------- Judge HONORABLE ERNEST J. DISANTIS, JR. ------------------------- Judge HONORABLE MICHAEL E. DUNLAVEY -------------------------- Judge HONORABLE ELIZABETH K. KELLY ----------------------------- Judge HONORABLE JOHN J. TRUCILLA --------------------------------- Judge Volume 85 TABLE OF CASES -A- Ager, et al. v. Steris Corporation ------------------------------------------------ 54 Alessi, et al. v. Millcreek Township Zoning Hearing Bd. and Sheetz, et al. 77 Altadonna; Commonwealth v. --------------------------------------------------- 90 American Manufacturers Mutual Insurance Co.; Odom v. ----------------- 232 Azzarello; Washam v. ------------------------------------------------------------ 181 -B- Beaton, et. al.; Brown v. ------------------------------------------------------------ -

Pennsylvania's Largest Employers (At Least 1,000 Employees)
Pennsylvania's Largest Employers (At Least 1,000 Employees) 1st Quarter, 2018 Combined Government Ownerships Center for Workforce Information & Analysis (877) 4WF-DATA • www.workstats.dli.pa.gov • [email protected] September 2018 Rank Employer Rank Employer 1 Federal Government 51 ACME Markets Inc 2 State Government 52 Aerotek Inc 3 Wal-Mart Associates Inc 53 Geisinger Medical Center 4 Trustees of the University of PA 54 Reading Hospital 5 City of Philadelphia 55 Dolgencorp LLC 6 Pennsylvania State University 56 Carnegie Mellon University 7 Giant Food Stores LLC 57 Abington Memorial Hospital 8 School District of Philadelphia 58 FedEx Ground Package System Inc 9 UPMC Presbyterian Shadyside 59 Highmark Inc 10 United Parcel Service Inc 60 Kohl's Department Stores Inc 11 PNC Bank NA 61 Rite Aid of Pennsylvania Inc 12 University of Pittsburgh 62 Marmaxx Operating Corporation 13 Lowe's Home Centers LLC 63 The Hershey Company 14 Weis Markets Inc 64 Wells Fargo NA 15 The Children's Hospital of Philadelphia 65 Temple University Hospital Inc 16 Comcast Cablevision Corp (PA) 66 York Hospital 17 Home Depot USA Inc 67 SmithKline Beecham Corporation 18 PA State System of Higher Education 68 Starbucks Corporation 19 Giant Eagle Inc 69 Boscov's Department Store LLC 20 Amazon.com DEDC LLC 70 School District of Pittsburgh 21 The Vanguard Group Inc 71 UPMC Pinnacle Hospitals 22 Target Corporation 72 Geisinger Clinic 23 Merck Sharp & Dohme Corporation 73 Dick's Sporting Goods Inc 24 Western Penn Allegheny Health 74 Hershey Entertainment & Resorts Co 25 -

Timeline of COVID-19 Relief for the Child Care Industry and Working
Timeline of COVID-19 Relief FIRST FIVE for the Child Care Industry and YEARS Working Families FUND The COVID-19 economic crisis has had a devastating impact on child care providers, causing widespread layoffs and closures nationwide. Significant declines in enrollment paired with steep increases in operating expenses have created an unsustainable financial situation for an industry that traditionally relies on razor-thin margins. Recognizing the essential role of child care for children, working families, and the economy, Democrats and Republicans in Congress have sought to prioritize funding and other relief opportunities for child care providers as part of ongoing COVID-19 recovery efforts. While Congress has not passed comprehensive economic stimulus legislation since March 2020, leaving families and businesses – including child care providers – without much-needed financial relief, every major federal pandemic recovery package introduced thus far has included dedicated funding for child care. The following is a high-level overview of the major COVID-19 recovery proposals and the provisions aimed at supporting child care providers, along with Congressional letters and resolutions calling for significant child care relief funding. Updated as of February 1, 2021 SUPPORTING AMERICA'S CHILD CARE PROVIDERS: COVID-19 ECONOMIC RECOVERY CARES Act Provides $3.5 Billion in Child Care Relief March 27, 2020: The largest enacted relief effort to date, which passed with broad bipartisan support, the Coronavirus Aid, Relief, and Economic Security (CARES) Act, MARCH included targeted support for the child care industry. The CARES Act provided the Child Care and Development Block Grant (CCDBG) with $3.5 billion and Head Start programs with $750 million. -
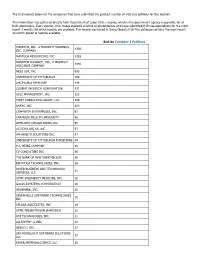
The H1B Records Below List the Companies That Have Submitted the Greatest Number of H1B Visa Petitions for This Location
The H1B records below list the companies that have submitted the greatest number of H1B visa petitions for this location. This information was gathered directly from Department of Labor (DOL) records, which is the government agency responsible for all H1B submissions. Every quarter, DOL makes available a listing of all companies who have submitted H1B visa applications for the most recent 3 months for which records are available. The records contained in Going Global's H1B Plus database contains the most recent 12-month period of records available. Sort by Company | Petitions MASTECH, INC., A MASTECH HOLDINGS, 4339 INC. COMPANY MASTECH RESOURCING, INC. 1393 MASTECH ALLIANCE, INC., A MASTECH 1040 HOLDINGS COMPANY NESS USA, INC. 693 UNIVERSITY OF PITTSBURGH 169 UHCP D/B/A UPMC MEP 144 COGENT INFOTECH CORPORATION 131 SDLC MANAGEMENT, INC. 123 FIRST CONSULTING GROUP, LLC 104 ANSYS, INC. 100 COMPUTER ENTERPRISES, INC. 97 CARNEGIE MELLON UNIVERSITY 96 INTELLECT DESIGN ARENA INC. 95 ACCION LABS US, INC. 63 HM HEALTH SOLUTIONS INC. 57 UNIVERSITY OF PITTSBURGH PHYSICIANS 44 H.J. HEINZ COMPANY 40 CV CONSULTING INC 36 THE BANK OF NEW YORK MELLON 36 INFOYUGA TECHNOLOGIES, INC. 36 BAYER BUSINESS AND TECHNOLOGY 31 SERVICES, LLC UPMC EMERGENCY MEDICINE, INC. 28 GALAX-ESYSTEMS CORPORATION 26 HIGHMARK, INC. 25 SEVEN HILLS SOFTWARE TECHNOLOGIES 25 INC VELAGA ASSOCIATES, INC 24 UPMC PRESBYTERIAN SHADYSIDE 22 DVI TECHNOLOGES, INC. 21 ALLEGHENY CLINIC 20 GENCO I. INC. 17 SRI MOONLIGHT SOFTWARE SOLUTIONS 17 LLC BAYER MATERIALSCIENCE, LLC 16 BAYER HEALTHCARE PHARMACEUTICALS, 16 INC. VISVERO, INC. 16 CYBYTE, INC. 15 BOMBARDIER TRANSPORTATION 15 (HOLDINGS) USA, INC. -

Federal Relief Overview
Federal Relief to Address the COVID-19 Pandemic To help keep you apprised of the various Federal relief packages to address the COVID-19 pandemic, please find below key information regarding the status, summary of key provisions, and other relevant information, generally in reverse chronological order. Biden Economic Recovery Legislation Status: Announced Biden is expected to propose a second economic recovery plan in the coming weeks that would address his longer- term job creation and development goals, which has been tagged as the Build Back Better plan. Biden Emergency Relief Legislation Status: Signed into law In January 2021, President Joe Biden has laid out the details of his $1.9 trillion emergency relief plan – the American Rescue Plan. The wide-ranging package aimed at containing the pandemic and supporting the economy includes: • increased direct payments to individual Americans, • increased unemployment insurance benefits, and • expanded medical and family leave. Congressional Democrats decided to employ the budgetary maneuver known as reconciliation to pass the American Rescue Plan. After establishing the budgetary rules for reconciliation as part of S.Con.Res.5, which passed both the House and Senate on February 5, the House passed the key legislation (H.R. 1319) in the early hours of Saturday, February 27 by a vote of 219 to 212. No Republicans voted in support of the bill, and two Democrats – Reps. Jared Golden (Maine) and Kurt Schrader (Ore.) – voted against it. Summaries of the legislation (by Committee) can be found here (Energy and Commerce), here (Ways and Means), here (Oversight and Government Reform), and here (Education and Labor). -

Download Our 2020 Corporate Profile
HIGHMARK HEALTH 2020 CORPORATE PROFILE 1 Living Health: Together for a Purpose 2020 Corporate Profile HIGHMARK HEALTH 2020 CORPORATE PROFILE 2 Our vision is a world Highmark Health is committed to reinventing the health experience so that everything and everyone works together for the health of those we serve. Boldly challenging unsustainable health care models of the past, we are where everyone building a new model, Living Health, to simplify the customer experience, free up clinicians to focus on care, embraces health. and leverage innovative technology and partnerships to deliver truly individualized health planning. We believe that creating a remarkable health experience will drive better health outcomes and lower total cost of care. Like our work, our financial priorities also serve our social purpose of building stronger communities of Our mission is to healthier people. In 2020, that included more than $750 million invested to support customers, providers, and create a remarkable communities during the pandemic, and another $760 million in capital investments. health experience, A DIVERSE PORTFOLIO OF LEADING HEALTH COMPANIES freeing people to Highmark Health’s diverse portfolio of aliates and subsidiaries meets a broad spectrum of health needs for be their best. consumers, business customers, and government entities. Highmark Inc. and its Blue-branded aliates HM Health Solutions combines technology and (Highmark Health Plans) proudly cover the leading industry knowledge to deliver business insurance needs of millions of individuals, families, solutions to health plan payers so they can run their and seniors, while also oering a variety of health- organizations eciently in a competitive and ever- related products and services. -

2019 Highmark Health Annual Report: Corporate Profile
HIGHMARK HEALTH 2019 CORPORATE PROFILE 1 Embracing Health in Remarkable Ways GLORIA, AHN PATIENT ▶ 2019 Corporate Profile HIGHMARK HEALTH 2019 CORPORATE PROFILE 2 Our vision is a world Highmark Health is committed to reinventing the health experience so that everything and everyone works together seamlessly for the health of those we serve. Boldly challenging unsustainable health care models of where everyone the past, we are simplifying the customer experience, freeing up clinicians to focus on care, and leveraging embraces health. innovative technology and partnerships to deliver truly individualized health planning. We believe that creating a remarkable health experience is also the path to better health outcomes and lower total cost of care. Like our work, our organizational giving — totaling $206.5 million in 2019 — centers on our larger social purpose of Our mission is to building stronger communities of healthier people. create a remarkable A DIVERSE PORTFOLIO OF LEADING HEALTH CARE COMPANIES health experience, Highmark Health’s strong and diverse portfolio of businesses meets a broad spectrum of health needs for freeing people to consumers, business customers, and government entities. be their best. Highmark Inc. and its Blue-branded affiliates (Health HM Home & Community Services specializes Plan) proudly cover the insurance needs of millions of in population health management solutions that individuals, families and seniors, while also offering a benefit payers, providers, and customers. variety of health-related products and services. HM Insurance Group works to protect businesses Allegheny Health Network (AHN) has more and their employees from the financial risks than 250 clinical facilities, including hospitals associated with catastrophic health care costs. -

Highmark Inc. Company Overview
Highmark Inc. Company Overview Vision A world where everyone embraces health Mission To create a remarkable health experience, freeing people to be their best Key Statistics • Founded Mid 1930s • Number of employees 6,000 • 5.6 million members are covered by Highmark Inc. • Highmark Health had $18 Billion in Operating Revenue in 2019 • Highmark Inc., together with its Blue-branded affiliates, collectively comprise the fourth-largest overall Blue Cross Blue Shield-affiliated organization in the country based on membership. Company Headquarters • Pittsburgh, Pa. Community Support • In 2019, the Health Plans' corporate giving benefited hundreds of organizations, donating more than $17 million in the areas of western, central, and northeastern Pennsylvania, West Virginia, and Delaware. In addition, Highmark's signature program, Walk for a Healthy Community, along with Adopt-a-School programs initiated by employees in Pittsburgh and Erie, and National Make a Difference Day of volunteering, provided significant benefits to individuals and the communities the Health Plans proudly serve. 1 Products • Highmark Inc. is a national, diversified health care partner based in Pittsburgh that serves members across the United States through its businesses in health insurance, dental insurance, and reinsurance. We offer a variety of plans in our core service areas. • Our diversified businesses also provide a broad spectrum of specialty products, such as dental insurance, and reinsurance to 50 million Americans across all 50 states and the District of Columbia. • Our diversified businesses rank among the nation's leaders in their categories: o Fifth largest dental insurance carrier in the U.S. o Among the top five largest stop loss carriers Awards • Medicare Advantage plans recognized for member satisfaction For the fourth consecutive year, Highmark's Medicare Advantage plans received high rankings in the annual J.D. -

Fifth Annual Father's Day Pledge Media Advisory
MEDIA ADVISORY Contact: Maggie Stasko, 412-642-7700 [email protected] Pittsburgh Says “NO MORE” to Domestic Violence and Sexual Assault 5th Annual Father’s Day Pledge Ceremony will take place at U.S. Steel Tower Plaza on June 13 – largest gathering to date expected with participation by several corporations WHAT: Father’s Day Pledge Public Signing Ceremony to End Gender Violence On Thursday, June 13, prominent business, civic and community leaders, and public officials will lend their voices to the prevention of domestic violence and sexual assault at the 5th annual Father’s Day Pledge Ceremony in downtown Pittsburgh. This year’s public gathering is expected to be the largest yet with groups from UPMC, Koppers, Ernst & Young LLC, PNC, KPMG, Highmark Health and several other organizations participating. During the rally, the leaders will recite and sign the pledge to end gender-based violence. The program will conclude with a public pledge signing. The event is hosted by Southwest PA Says No More, an initiative supported by FISA Foundation, The Heinz Endowments and United Way of Southwestern Pennsylvania. WHEN: Thursday, June 13, 2019, 12:00 p.m. to 1:00 p.m. WHERE: U.S. Steel Tower Plaza, 600 Grant Street, Downtown Pittsburgh (If it rains, the event will be held in the upper lobby of U.S. Steel Tower.) AGENDA: 12:00 p.m. Welcome by emcee Sally Wiggin 12:05 p.m. Opening remarks Kristy Trautmann, FISA Foundation Adam Baron, United Way of Southwestern PA 12:12 p.m. Prevention takes leadership The Honorable Rich Fitzgerald, County Executive The Honorable William Peduto, Mayor, City of Pittsburgh 12:20 p.m.