Microcystic Macular Changes in Primary Open-Angle Glaucoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Organization of the Inner Nuclear Layer of the Rabbit Retina
The Journal of Neuroscience. January 1995. 15(l): 875-888 The Organization of the Inner Nuclear Layer of the Rabbit Retina Enrica Strettoil and Richard H. Masland2 ‘Istituto di Neurofisiologia del C.N.R., Pisa, Italy; 2Program in Neuroscience and Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts The initial goal of this study was to establish an accounting function (reviewed by Kolb et al., 198 1; Masland, 1988; Cohen of the major classes of cells present in the inner nuclear and Sterling, 1990; WHssle and Boycott, 199 1; Masland, 1992). layer (INL) of the rabbit’s retina. Series of 80-100 radial What one does not know is how many more cell types there sections 1 pm thick were cut from retinal blocks dissected are-how many cells have not yet been encountered by current, at intervals along the vertical meridian. They were photo- essentially trial and error, methods. graphed at high magnification in the light microscope. By The missing cells-those invisible to present staining tech- visualizing the initial segments of processes leaving the so- niques-are important. They represent unseen actors in the ret- mata, we could identify each cell as a bipolar, amacrine, ina’s circuitry. The responses of the retinal ganglion cells are horizontal, or Muller cell. The identifications made by light controlled by all of the neurons afferent to them. If there are microscopy were confirmed by electron microscopy of al- unseen elements afferent to the ganglion cell, our understanding ternating ultrathin sections. On average, bipolar cells made of the creation of ganglion cell responses risks major error. -

Post-Cataract Cystoid Macular Oedema Prevention – Update 2019
Review Cystoid Macular Oedema Post-cataract Cystoid Macular Oedema Prevention – Update 2019 Andrzej Grzybowski,1,2 Reda Zemaitiene,3 Lina Mikalauskiene3 1. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland; 2. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland; 3. Department of Ophthalmology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania DOI: https://doi.org/10.17925/EOR.2019.13.1.37 seudophakic cystoid macular oedema (PCMO) is a common complication following both uncomplicated and complicated cataract surgery, becoming apparent about 6 weeks following surgery. PCMO may be asymptomatic in some cases, but in others is associated Pwith a reduction in visual acuity. The pathogenesis of PCMO is linked to postoperative inflammation and the release of inflammatory mediators. The use of topical steroids and/or nonsteroidal anti-inflammatory drugs can reduce the adverse effects of inflammation and have been used for the prevention and treatment of PCMO. However, the therapeutic effectiveness of these drugs is currently not well understood, partially because PCMO can spontaneously resolve as well as the multiple treatment protocols and the paucity of robust data exist. In this review we compare the various prophylactic options for PCMO and provide commentary on their efficacy. Keywords Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been Pseudophakic cystoid macular oedema, offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing post-operative inflammation, cataract surgery, postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract nonsteroidal anti-inflammatory drugs surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in Disclosure: Andrzej Grzybowski, Reda inclusion criteria, patient characteristics, prescription and duration of treatment. -

The Usefulness of Systemic Inflammatory Markers As Diagnostic
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE The usefulness of systemic inflammatory markers as diagnostic indicators of the pathogenesis of diabetic macular edema A utilidade de marcadores inflamatórios sistêmicos como indicadores diagnósticos da patogênese do edema macular diabético Cagri Ilhan1 , Mehmet Citirik2, Mehmet Murat Uzel3, Hasan Kiziltoprak4, Kemal Tekin5 1. Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey. 2. Department of Ophthalmology, University of Health Sciences, Ankara Ulucanlar Eye Education and Research Hospital, Ankara, Turkey. 3. Department of Ophthalmology, Balikesir University, Balikesir, Turkey. 4. Department of Ophthalmology, Bingol Maternity and Child Hospital, Bingol, Turkey. 5. Department of Ophthalmology, Ercis State Hospital, Van, Turkey. ABSTRACT | Purpose: To investigate the usefulness of sys- groups were similar (diabetic macular edema vs. non-diabetic temic inflammatory markers [i.e., white blood cell and platelet macular edema, p=0.08; diabetic macular edema vs. control, counts, mean platelet volume, and their ratios] as diagnostic p=0.02; and non- diabetic macular edema vs. control, p=0.78). markers of the pathogenesis of diabetic macular edema. Me- All other parameters were similar between groups (all p>0.05). thods: The study cohort included 80 diabetic macular edema Conclusion: The neutrophil/lymphocyte ratio and mean platelet patients (40 with diabetic retinopathy and 40 without) and 40 volume of the diabetic macular edema group were higher than healthy age- and sex-matched controls. Neutrophil, lymphocyte, those of the non-diabetic macular edema and control groups. monocyte, and platelet counts, and the mean platelet volume A neutrophil/lymphocyte ratio cutoff value of ≥2.26 was identified were determined from peripheral blood samples, and the as an indicator of the pathogenesis of diabetic macular edema monocyte/lymphocyte, platelet/lymphocyte, and mean platelet with high sensitivity and specificity. -

Radial and Tangential Dispersion Patterns in the Mouse Retina Are Cell
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2494-2498, March 1995 Neurobiology Radial and tangential dispersion patterns in the mouse retina are cell-class specific (cell migration/cell lineage/retinal development/transgenic mice/X chromosome inactivation) B. E. REESE*, A. R. HARvEyt, AND S.-S. TANt§ *Neuroscience Research Institute and Department of Psychology, University of California, Santa Barbara, CA 93106; tDepartment of Anatomy and Human Biology, University of Western Australia, Nedlands, WA 6009 Australia; and tDepartment of Anatomy and Cell Biology, University of Melbourne, Parkville, Victoria 3052, Australia Communicated by Pasko Rakic, Yale University School ofMedicine, New Haven, CT, December 16, 1994 ABSTRACT The retina is derived from a pseudostratified retinal cells remain clonally segregated, they should appear as germinal zone in which the relative position of a progenitor distinct groups of blue versus white cells. We have used this cell is believed to determine the position ofthe progeny aligned approach to address the issue of whether radially aligned cells in the radial axis. Such a developmental mechanism would in the mature retina reflect such a clonal derivation. ensure that radial arrays of cells which comprise functional units in the mature central nervous system are also clonally MATERIALS AND METHODS related. The present study has tested this hypothesis by using Retinas from adult transgenic mice, derived from founder line X chromosome-inactivation transgenic mosaic mice. We re- H253, which carries a lacZ transgene -

Paraneoplastic Retinopathy Associated with Metastatic Cutaneous Melanoma of Unknown Primary Site
PARANEOPLASTIC RETINOPATHY ASSOCIATED WITH METASTATIC CUTANEOUS MELANOMA OF UNKNOWN PRIMARY SITE l 2 l I HA AM KIRATLI , CHARLES E. THIRKILL , SEVGUL BILGI(: , BORA ELDEM YY 1 and ARMAN KE(:ECI Ankara, Turkey and Sacramento, California SUMMARY features of a patient with this rare syndrome are Purpose: To describe further the clinical and immuno described here. logical features of cutaneous melanoma-associated retinopathy, which is an infrequent form of paraneo CASE REPORT plastic syndrome. Methods: We studied the salient clinical and immuno A 66-year-old man without any prior systemic or logical aspects of a 66-year-old man with metastatic ocular problems presented with the complaint of cutaneous melanoma to lymph nodes of unknown mild visual loss of recent onset in his left eye. He had primary site who developed melanoma-associated experienced occasional flashing lights but had no retinopathy. difficulty with night vision. A few days earlier an Results: There was gradual loss of vision in the left eye. incisional biopsy had been done from his right Colour vision and night vision were not affected. Visual axillary region, where rapid enlargement of four or fields showed arcuate defects. A full-field electroretino five lymph nodes each measuring 3 X 2 X 2 cm was gram demonstrated attenuation of the b-wave ampli noticed. tude in the left eye. The a-wave was intact. Indirect His best corrected visual acuity was 6/9 in the right immunofluorescence techniques showed that the anti eye and 6/18 in the left eye. There was no afferent body reactions took place mainly in the outer plexiform pupillary defect. -

Optical Coherence Tomography Demonstrates Subretinal Macular Edema from Papilledema
CLINICAL SCIENCES Optical Coherence Tomography Demonstrates Subretinal Macular Edema From Papilledema Vincent J. Hoye III, MD; Audina M. Berrocal, MD; Thomas R. Hedges III, MD; Maria Luz Amaro-Quireza, OD Objective: To evaluate macular changes in eyes with duction in visual acuity. The subretinal fluid appeared papilledema from increased intracranial pressure using to arise from the peripapillary region, and all showed optical coherence tomography (OCT). some improvement in central vision as the fluid re- solved. Methods: Fifty-five patients with papilledema seen dur- ing 1998 and 1999 were studied with OCT of the optic Conclusions: Subretinal fluid accumulations can cause nerve and retinal nerve fiber layer. Nineteen of these also decreased visual acuity in patients with papilledema. Op- had OCT of the macula during periods of acute, sub- tical coherence tomography can demonstrate subretinal acute, or recurrent papilledema and were evaluated in fluid and can be used to follow the course of this impor- detail for this report. tant visual complication of papilledema. Results: Seven patients had OCT evidence of sub- retinal fluid involving the macula. All had some re- Arch Ophthalmol. 2001;119:1287-1290 OSS OF visual acuity from pap- mal findings on neuroimaging scans and illedema or optic nerve head elevated opening pressures on lumbar swelling due to increased in- puncture. Two patients had brain metas- tracranial pressure is uncom- tases, one from breast cancer and the other mon. The visual conse- from testicular cancer. Six patients were Lquences of papilledema usually are limited treated medically with acetazolamide to visual field defects and enlargement of and/or furosemide. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -
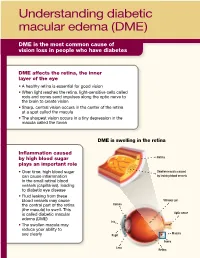
Understanding Diabetic Macular Edema (DME)
Understanding diabetic macular edema (DME) DME is the most common cause of vision loss in people who have diabetes DME affects the retina, the inner layer of the eye • A healthy retina is essential for good vision • When light reaches the retina, light-sensitive cells called rods and cones send impulses along the optic nerve to the brain to create vision • Sharp, central vision occurs in the center of the retina at a spot called the macula • The sharpest vision occurs in a tiny depression in the macula called the fovea DME is swelling in the retina Inflammation caused by high blood sugar Retina plays an important role • Over time, high blood sugar Swollen macula caused can cause inflammation by leaking blood vessels in the small retinal blood vessels (capillaries), leading to diabetic eye disease • Fluid leaking from these blood vessels may cause Vitreous gel Cornea the central part of the retina (the macula) to swell. This Optic nerve is called diabetic macular edema (DME) Iris • The swollen macula may reduce your ability to Macula see clearly Pupil Fovea Lens Retina Understanding DME (continued) Symptoms of DME • Early DME may not have any symptoms • As DME worsens, it can cause blurry central vision ranging from mild to severe • DME can cause significant vision loss over time How DME can affect vision Mild blurriness Moderate blurriness Severe blurriness Untreated DME can lead to vision loss • Talk to your doctor about treatment options • If you develop cataracts (hazy spots that can affect vision) in the lenses of your eyes, the natural lenses may be surgically removed and replaced with artificial ones • Having cataracts or artificial lenses can affect your treatment options ©2014 Allergan, Inc., Irvine, CA 92612 APC67SH14 141256. -

Primary Retinal Pathology in Multiple Sclerosis As Detected by Optical Coherence Tomography Downloaded From
doi:10.1093/brain/awq346 Brain 2011: 134; 518–533 | 518 BRAIN A JOURNAL OF NEUROLOGY Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography Downloaded from Shiv Saidha,1 Stephanie B. Syc,1 Mohamed A. Ibrahim,2 Christopher Eckstein,1 Christina V. Warner,1 Sheena K. Farrell,1 Jonathan D. Oakley,3 Mary K. Durbin,3 Scott A. Meyer,3 Laura J. Balcer,4 Elliot M. Frohman,5 Jason M. Rosenzweig,1 Scott D. Newsome,1 John brain.oxfordjournals.org N. Ratchford,1 Quan D. Nguyen2 and Peter A. Calabresi1 1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Carl Zeiss Meditec Inc, Dublin, CA, USA 4 Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA 5 Department of Neurology and Ophthalmology, University of Texas Southwestern, Dallas, TX, USA at University of Texas Southwestern Medical Center Dallas on February 23, 2011 Correspondence to: Dr Peter A. Calabresi, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 627, Baltimore, MD 21287, USA E-mail: [email protected] Optical coherence tomography studies in multiple sclerosis have primarily focused on evaluation of the retinal nerve fibre layer. The aetiology of retinal changes in multiple sclerosis is thought to be secondary to optic nerve demyelination. The objective of this study was to use optical coherence tomography to determine if a subset of patients with multiple sclerosis exhibit primary retinal neuronopathy, in the absence of retrograde degeneration of the retinal nerve fibre layer and to ascertain if such patients may have any distinguishing clinical characteristics. -

Introduction to Micropulse for Central Serous Chorioretinopathy
Advertorial MicroPulse for Central Serous Chorioretinopathy Sponsored by Quantel Medical Introduction to MicroPulse for Central Serous Chorioretinopathy MicroPulse laser has become one of our hospital’s standard treatments for CSCR. BY PROF. DR. SASCHA FAUSER, MD I would like to introduce you to the principle of One of the challenges with MicroPulse laser treatment is MicroPulse laser therapy. that you cannot visualize any laser effect during the treatment, To understand MicroPulse laser technology, we must which could give you the feeling that the treatment is not effec- first understand conventional photocoagulation. tive. Overall, the problem is overtreating, but undertreatment With conventional photocoagulation, the laser trans- can also be an issue. To avoid undertreating, surgeons should fers energy to the retina. The longer the laser beam is on, the more apply dense treatment and focus the laser carefully. The yel- heat is generated. This heat may result in tissue damage by coagu- low 577 nm wavelength has an advantage in these situations lating the proteins. It is similar to how an egg loses its transparency because, due to its absorption characteristics, it can be titrated. when heat is applied to it. Basically, you apply the laser to the periphery of the edematous We do not yet know the exact mechanism of action of laser, but area (in a nonedematous area) on low power that is increased one hypothesis is that the damaged photoreceptors are replaced by gradually until a just-visible effect appears. Once the thermal glial cells, which have less oxygen consumption, less vascular endo- threshold is determined for a patient, you reduce the power to thelial growth factor, less edema, and less neovascularization. -

Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy
Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy Mathias Abegg, MD, PhD,1 Muriel Dysli,1 Sebastian Wolf, MD, PhD,1 Jens Kowal, PhD,2 Pascal Dufour, MSc,2 Martin Zinkernagel, MD, PhD1 Purpose: To investigate retrograde axonal degeneration for its potential to cause microcystic macular edema (MME), a maculopathy that has been previously described in patients with demyelinating disease. To identify risk factors for MME and to expand the anatomic knowledge on MME. Design: Retrospective case series. Participants: We included 117 consecutive patients and 180 eyes with confirmed optic neuropathy of variable etiology. Patients with glaucoma were excluded. Methods: We determined age, sex, visual acuity, etiology of optic neuropathy, and the temporal and spatial characteristics of MME. Eyes with MME were compared with eyes with optic neuropathy alone and to healthy fellow eyes. With retinal layer segmentation we quantitatively measured the intraretinal anatomy. Main Outcome Measures: Demographic data, distribution of MME in the retina, and thickness of retinal layers were analyzed. Results: We found MME in 16 eyes (8.8%) from 9 patients, none of whom had multiple sclerosis or neu- romyelitis optica. The MME was restricted to the inner nuclear layer (INL) and had a characteristic perifoveal circular distribution. Compared with healthy controls, MME was associated with significant thinning of the ganglion cell layer and nerve fiber layer, as well as a thickening of the INL and the deeper retinal layers. Youth is a significant risk factor for MME. Conclusions: Microcystic macular edema is not specific for demyelinating disease. It is a sign of optic neuropathy irrespective of its etiology. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath.