DIABETIC MACULAR EDEMA a Review of Outcomes from Recent Studies on DME and How Those Findings Impact Clinical Treatment Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Cataract Cystoid Macular Oedema Prevention – Update 2019
Review Cystoid Macular Oedema Post-cataract Cystoid Macular Oedema Prevention – Update 2019 Andrzej Grzybowski,1,2 Reda Zemaitiene,3 Lina Mikalauskiene3 1. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland; 2. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland; 3. Department of Ophthalmology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania DOI: https://doi.org/10.17925/EOR.2019.13.1.37 seudophakic cystoid macular oedema (PCMO) is a common complication following both uncomplicated and complicated cataract surgery, becoming apparent about 6 weeks following surgery. PCMO may be asymptomatic in some cases, but in others is associated Pwith a reduction in visual acuity. The pathogenesis of PCMO is linked to postoperative inflammation and the release of inflammatory mediators. The use of topical steroids and/or nonsteroidal anti-inflammatory drugs can reduce the adverse effects of inflammation and have been used for the prevention and treatment of PCMO. However, the therapeutic effectiveness of these drugs is currently not well understood, partially because PCMO can spontaneously resolve as well as the multiple treatment protocols and the paucity of robust data exist. In this review we compare the various prophylactic options for PCMO and provide commentary on their efficacy. Keywords Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been Pseudophakic cystoid macular oedema, offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing post-operative inflammation, cataract surgery, postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract nonsteroidal anti-inflammatory drugs surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in Disclosure: Andrzej Grzybowski, Reda inclusion criteria, patient characteristics, prescription and duration of treatment. -

Incidence and Management of Acute Endophthalmitis After Intravitreal
Eye (2009) 23, 2187–2193 & 2009 Macmillan Publishers Limited All rights reserved 0950-222X/09 $32.00 www.nature.com/eye Incidence and O Artunay, E Yuzbasioglu, R Rasier, A Sengu¨l CLINICAL STUDY and H Bahcecioglu management of acute endophthalmitis after intravitreal bevacizumab (Avastin) injection Abstract recognition and treatment are key in maximizing outcomes in patients who Introduction The aim of this study was to developed endophthalmitis after intravitreal report the incidence and management of acute injection of bevacizumab. endophthalmitis after intravitreal injection of Eye (2009) 23, 2187–2193; doi:10.1038/eye.2009.7; Avastin (bevacizumab), and visual acuity published online 13 February 2009 outcomes of three eyes of three patients who developed acute endophthalmitis following Keywords: intravitreal injection; bevacizumab; intravitreal injection of Avastin. endophthalmitis Methods This clinical retrospective, non-comparative study included 3022 Introduction intravitreal injections of 1.25 mg bevacizumab consecutively performed for 1822 eyes with Intravitreal injection of medications is becoming exudative age-related macular degeneration increasingly accepted for treatment of various and other retinal diseases. Of 3022 injections, retinal disorders, with effective intravitreal 1200 were reinjections. After clinical therapies being commonly administered in the appearance of post-injection endophthalmitis, vitreoretinal clinical or surgical environment. Department of immediate intervention was performed, With the increase in -

Endophthalmitis After Intravitreal Injections in Patients with Self-Reported Iodine Allergy
Endophthalmitis After Intravitreal Injections in Patients With Self-reported Iodine Allergy BOBECK S. MODJTAHEDI, TAVE´ VAN ZYL, HEMANG K. PANDYA, ROBERT E. LEONARD, II, AND DEAN ELIOTT PURPOSE: To present cases of endophthalmitis IMITING THE RISK OF POSTINJECTION ENDOPHTHAL- following intravitreal injections where povidone-iodine mitis is an area of considerable practical and aca- (PI) was not used as part of the surgical preparation. demic interest, especially in the era of regular L DESIGN: Retrospective case series. intravitreal injections. Although the incidence of postin- METHODS: All cases of presumed injection-related jection endophthalmitis is low (0.056% per injection in a endophthalmitis presenting to the Massachusetts Eye recent meta-analysis),1 the risk to an individual patient is and Ear Infirmary between June 2008 and November magnified by the recurrent nature of the procedure. Using 2014 and Dean McGee Eye Institute between January povidone-iodine (PI) for surgical antisepsis is well estab- 2010 and January 2015 were identified. Patients who lished and is the only preoperative measure shown to did not receive PI preparation owing to documented reduce the risk of endophthalmitis in patients undergoing self-reported allergy to iodine, iodine-containing contrast intraocular surgery.2 The importance of PI application material, or shellfish were identified and their injection prior to intravitreal injection has been observed by histories and clinical courses reviewed. Nentwich and associates,3 Bhavsar and Sandler,4 and Bryn- 5 RESULTS: The combined rate of postinjection endoph- skov and associates, the latter of whom described no cases thalmitis at these 2 centers was 0.019%. Among 42 of endophthalmitis after 20 293 injections. -

The Usefulness of Systemic Inflammatory Markers As Diagnostic
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE The usefulness of systemic inflammatory markers as diagnostic indicators of the pathogenesis of diabetic macular edema A utilidade de marcadores inflamatórios sistêmicos como indicadores diagnósticos da patogênese do edema macular diabético Cagri Ilhan1 , Mehmet Citirik2, Mehmet Murat Uzel3, Hasan Kiziltoprak4, Kemal Tekin5 1. Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey. 2. Department of Ophthalmology, University of Health Sciences, Ankara Ulucanlar Eye Education and Research Hospital, Ankara, Turkey. 3. Department of Ophthalmology, Balikesir University, Balikesir, Turkey. 4. Department of Ophthalmology, Bingol Maternity and Child Hospital, Bingol, Turkey. 5. Department of Ophthalmology, Ercis State Hospital, Van, Turkey. ABSTRACT | Purpose: To investigate the usefulness of sys- groups were similar (diabetic macular edema vs. non-diabetic temic inflammatory markers [i.e., white blood cell and platelet macular edema, p=0.08; diabetic macular edema vs. control, counts, mean platelet volume, and their ratios] as diagnostic p=0.02; and non- diabetic macular edema vs. control, p=0.78). markers of the pathogenesis of diabetic macular edema. Me- All other parameters were similar between groups (all p>0.05). thods: The study cohort included 80 diabetic macular edema Conclusion: The neutrophil/lymphocyte ratio and mean platelet patients (40 with diabetic retinopathy and 40 without) and 40 volume of the diabetic macular edema group were higher than healthy age- and sex-matched controls. Neutrophil, lymphocyte, those of the non-diabetic macular edema and control groups. monocyte, and platelet counts, and the mean platelet volume A neutrophil/lymphocyte ratio cutoff value of ≥2.26 was identified were determined from peripheral blood samples, and the as an indicator of the pathogenesis of diabetic macular edema monocyte/lymphocyte, platelet/lymphocyte, and mean platelet with high sensitivity and specificity. -

Intravitreal Angiogenesis Inhibitors for Retinal Vascular Conditions
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 06/01/19 SECTION: DRUGS LAST REVIEW DATE: 04/16/19 LAST CRITERIA REVISION DATE: ARCHIVE DATE: LUCENTIS® (ranibizumab) for intravitreal injection Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Medical Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Medical Coverage Guidelines are subject to change as new information becomes available. For purposes of this Medical Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. -

Intravitreal Injection Procedure Instructional Outline
Intravitreal Injection Procedure Instructional Outline Anh-Danh T. Phan, M.D. Indiana University School of Medicine Department of Ophthalmology Intravitreal Injection Procedure Instructional Outline Anh-Danh T. Phan, M.D. Assistant Professor of Ophthalmology Retina and Vitreous Service Indiana University School of Medicine Department of Ophthalmology / Glick Eye Institute Indianapolis, IN Email: [email protected] Background Statement: Intravitreal injection is the most common procedure in ophthalmology, yet carries associated risks. Mastery of the procedure particularly during residency training is critical to address the staggering patient treatment needs. Objectives: To transfer, along with accompanying instructional video, useful knowledge and skills for performing the intravitreal injection during ophthalmology training, enabling residents to understand: (1) the precautions before, during, and after the procedure, including risk of endophthalmitis; (2) the technique performed at a major university medical center; and (3) a method to standardize the procedure across multiple clinical settings. Residents are encouraged to gather instructional input from their supervising retinal specialists during training to develop their own procedural approach most comfortable, while observing the underlying principles and concepts outlined herein. Conflict of Interest The author has no propriety interest in either the outline or its subject matter. Legal Disclaimer The author provides this material for educational purposes only. It is not intended -

Optical Coherence Tomography Demonstrates Subretinal Macular Edema from Papilledema
CLINICAL SCIENCES Optical Coherence Tomography Demonstrates Subretinal Macular Edema From Papilledema Vincent J. Hoye III, MD; Audina M. Berrocal, MD; Thomas R. Hedges III, MD; Maria Luz Amaro-Quireza, OD Objective: To evaluate macular changes in eyes with duction in visual acuity. The subretinal fluid appeared papilledema from increased intracranial pressure using to arise from the peripapillary region, and all showed optical coherence tomography (OCT). some improvement in central vision as the fluid re- solved. Methods: Fifty-five patients with papilledema seen dur- ing 1998 and 1999 were studied with OCT of the optic Conclusions: Subretinal fluid accumulations can cause nerve and retinal nerve fiber layer. Nineteen of these also decreased visual acuity in patients with papilledema. Op- had OCT of the macula during periods of acute, sub- tical coherence tomography can demonstrate subretinal acute, or recurrent papilledema and were evaluated in fluid and can be used to follow the course of this impor- detail for this report. tant visual complication of papilledema. Results: Seven patients had OCT evidence of sub- retinal fluid involving the macula. All had some re- Arch Ophthalmol. 2001;119:1287-1290 OSS OF visual acuity from pap- mal findings on neuroimaging scans and illedema or optic nerve head elevated opening pressures on lumbar swelling due to increased in- puncture. Two patients had brain metas- tracranial pressure is uncom- tases, one from breast cancer and the other mon. The visual conse- from testicular cancer. Six patients were Lquences of papilledema usually are limited treated medically with acetazolamide to visual field defects and enlargement of and/or furosemide. -

Informed Consent for Lucentis Tm (Ranibizumab) Intravitreal Injection
PATIENT _________________________________________DATE __________________ OD OS INFORMED CONSENT FOR LUCENTIS TM (RANIBIZUMAB) INTRAVITREAL INJECTION INDICATIONS Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 years of age. There are two types of macular degeneration: dry and wet. In the “wet” form of AMD, abnormal blood vessels grow in the back of the eye. Sometimes these vessels leak blood or fluid that causes blurred or distorted vision. Without treatment, vision loss may be quick and severe. POSSIBLE BENEFITS, LIMITATIONS, AND ADMINISTRATION Lucentis TM works by inhibiting the growth of the abnormal blood vessels that cause AMD. It is also used to treat swelling of the macula due to MAD. The goal of treatment is to prevent further loss of vision. Although some patients have regained vision, the medication may not restore vision that has already been lost, and may not ultimately prevent further loss of vision caused by the disease. After the pupil is dilated and the eye is numbed with anesthesia, the medication is injected into the vitreous or jelly-like substance in the back chamber of the eye. Lucentis TM is administered by an injection into your eye as needed at regular intervals (about every four weeks); your ophthalmologist will tell you how often you will receive the injection, and for how long. ALTERNATIVES You do not have to receive treatment for your condition, although without treatment, AMD can lead to further vision loss and blindness, sometimes very quickly. Other forms of treatment are available. At present, there are two other FDA-approved treatments for neovascular AMD: Photodynamic therapy with a drug called Visudyne TM and injection into the eye of a drug called Macugen TM . -

Intravitreal Injections
Intravitreal Injections This material will help you understand your intravitreal injection treatment and what you can expect. What are intravitreal injections? An intravitreal injection (IVI) is a shot of medicine into your eye using a thin needle. The inside of your eye is filled with a gel-like fluid called the vitreous (vit-ree-us). The doctor or nurse will inject the medicine through the front of the eye into the vitreous, near the retina at the back of the eye (see picture on right). Image courtesy of the Collaborative Ocular Melanoma Study Group The medicine used will depend on your condition being treated. IVIs are used to treat many eye problems, including macular degeneration, diabetic retinopathy, and some cases of eye cancer. What can I expect before my treatment? There are no special preparations for IVI. You should eat normally and take all your regular medicines before you come in. Let your doctor know any medications you are currently taking, as well as any allergies you have. Kellogg Eye Center Intravitreal Injections 1 This treatment is performed in your doctor’s office, so you will be able to go home the same day. If you do not feel comfortable driving after your treatment, you may want to bring a friend or family member with you to drive you home. What can I expect on the day of my treatment? On the day of your IVI, you will come to the Kellogg Eye Center Oncology Clinic. First, you will be given eye drops to dilate (widen) your pupils. Your doctor will then have you lie down in a comfortable face-up position. -

Ocular Anti-VEGF Therapy for Diabetic Retinopathy
900 Diabetes Care Volume 37, April 2014 Ning Cheung,1,2,3 Ian Y. Wong,1 and Ocular Anti-VEGF Therapy for Tien Y. Wong2,3 BENCH TO CLINIC SYMPOSIA Diabetic Retinopathy: Overview of Clinical Efficacy and Evolving Applications Diabetes Care 2014;37:900–905 | DOI: 10.2337/dc13-1990 Ocular anti-vascular endothelial growth factor (VEGF) therapy represents one of the most significant advances in modern medicine. The introduction and widespread use of ocular anti-VEGF therapy for age-related macular degenera- tion heralded a new era in the treatment of vascular and exudative diseases of the retina. Its expanding indications now include diabetic macular edema and proliferative diabetic retinopathy, two vision-threatening forms of diabetic retinopathy. It is widely anticipated that ocular anti-VEGF therapy could spark a dramatic shift in the treatment paradigm for diabetic retinopathy. However, despite its clear efficacy shown in clinical trials, the dynamic landscape of evolving medical, ethical, and economic issues related to this new treatment suggests significant challenges ahead. In this article, we provide a discussion of this topic as part of this two-part Bench to Clinic narrative. Here, our Clinic contribution provides an overview of the current evidence from clinical trials on anti-VEGF therapy for diabetic retinopathy, and highlights the hopes and fears of this new treatment from clinical and public health standpoints. In the Bench narrative that precedes this contribution, Simo´ et al. provide an overview of the role of VEGF in the pathogenesis of diabetic retinopathy. Ocular anti-vascular endothelial growth factor (VEGF) therapy represents one of the most significant advances in modern medicine. -
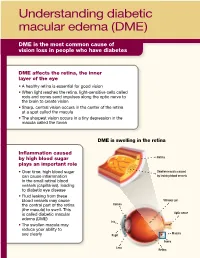
Understanding Diabetic Macular Edema (DME)
Understanding diabetic macular edema (DME) DME is the most common cause of vision loss in people who have diabetes DME affects the retina, the inner layer of the eye • A healthy retina is essential for good vision • When light reaches the retina, light-sensitive cells called rods and cones send impulses along the optic nerve to the brain to create vision • Sharp, central vision occurs in the center of the retina at a spot called the macula • The sharpest vision occurs in a tiny depression in the macula called the fovea DME is swelling in the retina Inflammation caused by high blood sugar Retina plays an important role • Over time, high blood sugar Swollen macula caused can cause inflammation by leaking blood vessels in the small retinal blood vessels (capillaries), leading to diabetic eye disease • Fluid leaking from these blood vessels may cause Vitreous gel Cornea the central part of the retina (the macula) to swell. This Optic nerve is called diabetic macular edema (DME) Iris • The swollen macula may reduce your ability to Macula see clearly Pupil Fovea Lens Retina Understanding DME (continued) Symptoms of DME • Early DME may not have any symptoms • As DME worsens, it can cause blurry central vision ranging from mild to severe • DME can cause significant vision loss over time How DME can affect vision Mild blurriness Moderate blurriness Severe blurriness Untreated DME can lead to vision loss • Talk to your doctor about treatment options • If you develop cataracts (hazy spots that can affect vision) in the lenses of your eyes, the natural lenses may be surgically removed and replaced with artificial ones • Having cataracts or artificial lenses can affect your treatment options ©2014 Allergan, Inc., Irvine, CA 92612 APC67SH14 141256. -

The Role of Topical Antibiotic Prophylaxis to Prevent Endophthalmitis After Intravitreal Injection
The Role of Topical Antibiotic Prophylaxis to Prevent Endophthalmitis after Intravitreal Injection Philip Storey, MD, MPH,1 Michael Dollin, MD,1 John Pitcher, MD,1 Sahitya Reddy, BA,2 Joseph Vojtko, BA,2 James Vander, MD,1 Jason Hsu, MD,1 Sunir J. Garg, MD,1for the Post-Injection Endophthalmitis Study Team* Objective: To compare the incidence of endophthalmitis after intravitreal injection with and without topical postinjection antibiotic prophylaxis. Design: Retrospective case-control study. Participants: All patients treated with intravitreal injection of ranibizumab, bevacizumab, or aflibercept for a variety of retinal vascular diseases at a single, large retina practice between January 1, 2009, and October 1, 2012, were included. Methods: The total numbers of patients and injections were determined from a review of billing code and practice management records. Endophthalmitis cases were determined from billing records and from an infection log. All cases of endophthalmitis were confirmed with chart review. A 28-month period when topical antibiotics were prescribed after intravitreal injection was compared with a 9-month period when topical antibiotics were not prescribed. Patients treated during an 8-month transition period were excluded to allow for the conversion of antibiotic prescription practices. Main Outcome Measures: Incidence of endophthalmitis, visual acuity outcomes, and microbial spectrum. Results: During the study period, a total of 117 171 intravitreal injections were performed (57 654 injections during the topical antibiotic period, 24 617 during the transition period, and 34 900 during the no-antibiotic period), with a total of 44 cases of suspected endophthalmitis (0.038%; 1 in 2663 injections), 17 of which showed culture-positive results (0.015%; 1 in 6892 injections).