Optical Coherence Tomography Demonstrates Subretinal Macular Edema from Papilledema
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Cataract Cystoid Macular Oedema Prevention – Update 2019
Review Cystoid Macular Oedema Post-cataract Cystoid Macular Oedema Prevention – Update 2019 Andrzej Grzybowski,1,2 Reda Zemaitiene,3 Lina Mikalauskiene3 1. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland; 2. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland; 3. Department of Ophthalmology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania DOI: https://doi.org/10.17925/EOR.2019.13.1.37 seudophakic cystoid macular oedema (PCMO) is a common complication following both uncomplicated and complicated cataract surgery, becoming apparent about 6 weeks following surgery. PCMO may be asymptomatic in some cases, but in others is associated Pwith a reduction in visual acuity. The pathogenesis of PCMO is linked to postoperative inflammation and the release of inflammatory mediators. The use of topical steroids and/or nonsteroidal anti-inflammatory drugs can reduce the adverse effects of inflammation and have been used for the prevention and treatment of PCMO. However, the therapeutic effectiveness of these drugs is currently not well understood, partially because PCMO can spontaneously resolve as well as the multiple treatment protocols and the paucity of robust data exist. In this review we compare the various prophylactic options for PCMO and provide commentary on their efficacy. Keywords Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been Pseudophakic cystoid macular oedema, offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing post-operative inflammation, cataract surgery, postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract nonsteroidal anti-inflammatory drugs surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in Disclosure: Andrzej Grzybowski, Reda inclusion criteria, patient characteristics, prescription and duration of treatment. -

The Usefulness of Systemic Inflammatory Markers As Diagnostic
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE The usefulness of systemic inflammatory markers as diagnostic indicators of the pathogenesis of diabetic macular edema A utilidade de marcadores inflamatórios sistêmicos como indicadores diagnósticos da patogênese do edema macular diabético Cagri Ilhan1 , Mehmet Citirik2, Mehmet Murat Uzel3, Hasan Kiziltoprak4, Kemal Tekin5 1. Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey. 2. Department of Ophthalmology, University of Health Sciences, Ankara Ulucanlar Eye Education and Research Hospital, Ankara, Turkey. 3. Department of Ophthalmology, Balikesir University, Balikesir, Turkey. 4. Department of Ophthalmology, Bingol Maternity and Child Hospital, Bingol, Turkey. 5. Department of Ophthalmology, Ercis State Hospital, Van, Turkey. ABSTRACT | Purpose: To investigate the usefulness of sys- groups were similar (diabetic macular edema vs. non-diabetic temic inflammatory markers [i.e., white blood cell and platelet macular edema, p=0.08; diabetic macular edema vs. control, counts, mean platelet volume, and their ratios] as diagnostic p=0.02; and non- diabetic macular edema vs. control, p=0.78). markers of the pathogenesis of diabetic macular edema. Me- All other parameters were similar between groups (all p>0.05). thods: The study cohort included 80 diabetic macular edema Conclusion: The neutrophil/lymphocyte ratio and mean platelet patients (40 with diabetic retinopathy and 40 without) and 40 volume of the diabetic macular edema group were higher than healthy age- and sex-matched controls. Neutrophil, lymphocyte, those of the non-diabetic macular edema and control groups. monocyte, and platelet counts, and the mean platelet volume A neutrophil/lymphocyte ratio cutoff value of ≥2.26 was identified were determined from peripheral blood samples, and the as an indicator of the pathogenesis of diabetic macular edema monocyte/lymphocyte, platelet/lymphocyte, and mean platelet with high sensitivity and specificity. -
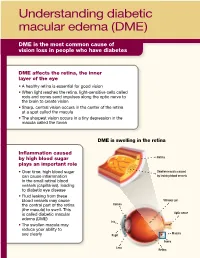
Understanding Diabetic Macular Edema (DME)
Understanding diabetic macular edema (DME) DME is the most common cause of vision loss in people who have diabetes DME affects the retina, the inner layer of the eye • A healthy retina is essential for good vision • When light reaches the retina, light-sensitive cells called rods and cones send impulses along the optic nerve to the brain to create vision • Sharp, central vision occurs in the center of the retina at a spot called the macula • The sharpest vision occurs in a tiny depression in the macula called the fovea DME is swelling in the retina Inflammation caused by high blood sugar Retina plays an important role • Over time, high blood sugar Swollen macula caused can cause inflammation by leaking blood vessels in the small retinal blood vessels (capillaries), leading to diabetic eye disease • Fluid leaking from these blood vessels may cause Vitreous gel Cornea the central part of the retina (the macula) to swell. This Optic nerve is called diabetic macular edema (DME) Iris • The swollen macula may reduce your ability to Macula see clearly Pupil Fovea Lens Retina Understanding DME (continued) Symptoms of DME • Early DME may not have any symptoms • As DME worsens, it can cause blurry central vision ranging from mild to severe • DME can cause significant vision loss over time How DME can affect vision Mild blurriness Moderate blurriness Severe blurriness Untreated DME can lead to vision loss • Talk to your doctor about treatment options • If you develop cataracts (hazy spots that can affect vision) in the lenses of your eyes, the natural lenses may be surgically removed and replaced with artificial ones • Having cataracts or artificial lenses can affect your treatment options ©2014 Allergan, Inc., Irvine, CA 92612 APC67SH14 141256. -

Introduction to Micropulse for Central Serous Chorioretinopathy
Advertorial MicroPulse for Central Serous Chorioretinopathy Sponsored by Quantel Medical Introduction to MicroPulse for Central Serous Chorioretinopathy MicroPulse laser has become one of our hospital’s standard treatments for CSCR. BY PROF. DR. SASCHA FAUSER, MD I would like to introduce you to the principle of One of the challenges with MicroPulse laser treatment is MicroPulse laser therapy. that you cannot visualize any laser effect during the treatment, To understand MicroPulse laser technology, we must which could give you the feeling that the treatment is not effec- first understand conventional photocoagulation. tive. Overall, the problem is overtreating, but undertreatment With conventional photocoagulation, the laser trans- can also be an issue. To avoid undertreating, surgeons should fers energy to the retina. The longer the laser beam is on, the more apply dense treatment and focus the laser carefully. The yel- heat is generated. This heat may result in tissue damage by coagu- low 577 nm wavelength has an advantage in these situations lating the proteins. It is similar to how an egg loses its transparency because, due to its absorption characteristics, it can be titrated. when heat is applied to it. Basically, you apply the laser to the periphery of the edematous We do not yet know the exact mechanism of action of laser, but area (in a nonedematous area) on low power that is increased one hypothesis is that the damaged photoreceptors are replaced by gradually until a just-visible effect appears. Once the thermal glial cells, which have less oxygen consumption, less vascular endo- threshold is determined for a patient, you reduce the power to thelial growth factor, less edema, and less neovascularization. -

Diabetic Retinopathy What You Should Know
Diabetic Retinopathy What You Should Know U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Eye Institute The National Eye Institute (NEI) conducts and supports research that leads to sight-saving treatments and plays a key role in reducing visual impairment and blindness. NEI is part of the National Institutes of Health, an agency of the U.S. Department of Health and Human Services. For more information, contact— National Eye Institute National Institutes of Health 2020 Vision Place Bethesda, MD 20892–3655 Telephone: 301–496–5248 Email: [email protected] Website: www.nei.nih.gov 2 Contents About Diabetic Retinopathy 1 Detection 3 Prevention and Treatment 5 What You Can Do 10 Current Research 12 Additional Resources 13 4 4 About Diabetic Retinopathy What is Diabetic Retinopathy? Diabetic retinopathy is a complication of diabetes and the leading cause of vision impairment and blindness among working-age adults. It occurs when diabetes damages the tiny blood vessels in the retina, which is the light-sensitive tissue at the back of the eye. Diabetic retinopathy may lead to diabetic macular edema (DME), which is a swelling in an area of the retina called the macula. Diabetic retinopathy involves damage to the retina, the light-sensitive tissue at the back of the eye. What causes diabetic retinopathy? Chronically high blood sugar from diabetes is associated with damage to the tiny blood vessels in the retina, leading to diabetic retinopathy. The retina detects light and converts it to signals sent through the optic nerve to the brain. Diabetic retinopathy can cause blood vessels in the retina to leak fluid 1 or hemorrhage (bleed), distorting vision. -

Intraocular Pressure Elevations Are Not Necessarily Glaucoma: Perspectives on OZURDEX (Dexamethasone Intravitreal Implant) 0.7 Mg Treatment
Supplement to May/June 2017 Sponsored by Intraocular Pressure Elevations Are Not Necessarily Glaucoma: Perspectives on OZURDEX (dexamethasone intravitreal implant) 0.7 mg Treatment A discussion on the differences between elevated intraocular pressure (IOP) and glaucoma, and how to assess each in patients with diabetic macular edema. Indications and Usage IMPORTANT SAFETY INFORMATION Diabetic Macular Edema Contraindications OZURDEX® (dexamethasone intravitreal implant) is a Ocular or Periocular Infections: OZURDEX® corticosteroid indicated for the treatment of diabetic (dexamethasone intravitreal implant) is contraindicated macular edema. in patients with active or suspected ocular or periocular infections including most viral diseases of the cornea Retinal Vein Occlusion and conjunctiva, including active epithelial herpes OZURDEX® is a corticosteroid indicated for the treatment simplex keratitis (dendritic keratitis), vaccinia, varicella, of macular edema following branch retinal vein occlusion mycobacterial infections, and fungal diseases. (BRVO) or central retinal vein occlusion (CRVO). Please see additional Important Safety Posterior Segment Uveitis Information on the following pages. OZURDEX® is indicated for the treatment of noninfectious uveitis affecting the posterior segment of the eye. PERSPECTIVES ON OZURDEX (DEXAMETHASONE INTRAVITREAL IMPLANT) 0.7 MG TREATMENT Intraocular Pressure Elevations Are Not Necessarily Glaucoma: Perspectives on OZURDEX Treatment A discussion on the differences between elevated IOP and glaucoma, and -

DIABETIC MACULAR EDEMA a Review of Outcomes from Recent Studies on DME and How Those Findings Impact Clinical Treatment Patterns
Insert to July/August 2016 AN UPDATE ON THE LATEST CLINICAL TRIALS IN DIABETIC MACULAR EDEMA A review of outcomes from recent studies on DME and how those findings impact clinical treatment patterns. Supported via advertising by Regeneron and Notal Vision Discover Strength in ef cacy As demonstrated in phase 3 clinical trials in patients with Wet AMD, Macular Edema following RVO, DME, and DR in Patients with DME Choose EYLEA® (a ibercept) Injection from the start Learn about EYLEA at EYLEA.us/rt INDICATIONS AND IMPORTANT SAFETY INFORMATION INDICATIONS EYLEA® (a ibercept) Injection is indicated for the treatment There is a potential risk of arterial thromboembolic events of patients with Neovascular (Wet) Age-related Macular (ATEs) following intravitreal use of VEGF inhibitors, including Degeneration (AMD), Macular Edema following Retinal Vein EYLEA. ATEs are de ned as nonfatal stroke, nonfatal Occlusion (RVO), Diabetic Macular Edema (DME), and Diabetic myocardial infarction, or vascular death (including deaths of Retinopathy (DR) in Patients with DME. unknown cause). The incidence of reported thromboembolic events in wet AMD studies during the rst year was 1.8% CONTRAINDICATIONS (32 out of 1824) in the combined group of patients treated ® EYLEA (a ibercept) Injection is contraindicated in patients with EYLEA. The incidence in the DME studies from baseline with ocular or periocular infections, active intraocular to week 52 was 3.3% (19 out of 578) in the combined group in ammation, or known hypersensitivity to a ibercept or of patients treated with EYLEA compared with 2.8% (8 out to any of the excipients in EYLEA. of 287) in the control group; from baseline to week 100, WARNINGS AND PRECAUTIONS the incidence was 6.4% (37 out of 578) in the combined Intravitreal injections, including those with EYLEA, have been group of patients treated with EYLEA compared with 4.2% associated with endophthalmitis and retinal detachments. -

The Diagnostic Dilemma of Pseudopapilledema
The Diagnostic Dilemma of Pseudopapilledema Tiffenie Harris, OD, FAAO Associate Professor Western University College of Optometry Author’s Bio Dr. Harris is a graduate of Indiana University School of Optometry. She practiced primary care optometry in the Detroit Metropolitan Area for 10 years prior to starting her academic career in 2004 at her Alma matter. She joined Western University College of Optometry in 2008. Dr. Harris is the Course Director for Principles and Practice of Anterior Segment and Posterior Segment courses. Diagnostic Dilemma Optic nerve head (ONH) elevation tends to be the most intimidating ocular finding especially when it presents bilaterally. The purpose of this article is to provide the clinician with clinical strategies that will enhance their assessment and management of bilateral disc elevations. In addition, this topic is important to review because effective management will reduce over-referrals for neurological evaluations, thus decreasing health care costs while avoiding needless and expensive neurological testing. The optometrist’s role includes detection of disease with a timely and appropriate referral to specialists as well as co-management and/or monitoring once under the care of a physician. A clinical case in which the optic nerves are severely swollen is easy to diagnose. Yet, a case in which the nerves appear to be mildly or moderately elevated or swollen makes the diagnosis more challenging, especially when the patient presents with symptoms of headaches. This type of quandary frequently can cause alarm for the clinician, which often eclipses the fact that the patient has no other signs or symptoms of increased intracranial pressure (ICP). -

Intravitreal Triamcinolone
CLINICAL SCIENCES Acute Endophthalmitis Incidence Intravitreal Triamcinolone Andrew C. Westfall, MD; Alexander Osborn, PhD; Derek Kuhl, PhD, MD; Matthew S. Benz, MD; William F. Mieler, MD; Eric R. Holz, MD Objective: To report the incidence of acute postinjec- cedure. One patient developed acute, culture-negative, tion endophthalmitis following intravitreal injection of postoperative endophthalmitis 4 days after receiving IVTA, triamcinolone acetonide (IVTA) as an office procedure. resulting in an incidence of 0.10%. In this case, the pre- senting symptoms were decreased vision and acute con- Methods: Retrospective, noncomparative, consecu- junctival erythema. The case was notable for the ab- tive, interventional case series of all patients who had re- sence of pain or hypopyon. ceived IVTA at 2 clinical centers between January 1, 2000, and January 30, 2004. Conclusion: Although acute postoperative endophthal- mitis may follow IVTA, our experience suggests that this Results: A total of 1006 eyes received IVTA. None of is a relatively uncommon event. the eyes developed acute, culture-positive, postopera- tive endophthalmitis in the 6 weeks following the pro- Arch Ophthalmol. 2005;123:1075-1077 HE INTRAVITREAL USE OF TRI- ing IVTA injection between January 1, 2000, and amcinolone acetonide January 30, 2004, at 2 centers (Baylor College (IVTA) has become in- of Medicine, Houston, Tex, and Austin Retina creasingly popular. Indica- Associates, College Station, Tex). The indica- tions for injection either tions for injection included diabetic macular edema, pseudophakic macular edema, edema alone or in combination with photody- T due to retinal vein occlusion, uveitic macular namic therapy include macular edema due edema, and exudative age-related maculopa- to diabetes mellitus, retinal vein occlu- thy. -

Towards Managing and Controlling Aflatoxin Producers Within
OPEN ACCESS Freely available online Journal of Eye Diseases and Disorders Short Communication Macular Edema build-up of Fluid in Macula Menakshi Chaku1*, William C. Stewart2 1Department of Ophthalmology, Boston Medical Center/Boston University School of Medicine, Boston, MA 02118, USA; 2Department of PRN Pharma Farm, University of Colorado State, Las Vegas, USA DESCRIPTION to VEGF Medication is the treatment depends on enemy of VEGF drugs, which are directed to the harmed eye through an Macula is the focal area of the retina (established by the light exceptionally slim needle. Hostile to VEGF (against Vascular delicate tissues situated at the rear of the eye, wherein macula Endothelial Development Factor) drugs are intended to check the inside the retina serves for sharp, straight-ahead vision) and development of anomalous veins in your retina, in this manner undesirable development of liquids there is eluded as the "Macular forestalling undesirable liquid spillage. Vitrectomy surgery is some Edema". Any aggregation of liquids inside the macula brings about of the time; macula goes through glassy pulling, which results in expanding and thickening of the macula, which prompts twisted macular edema (glassy is the jam like substance filling the rear of vision. the eye). Under such conditions, prepared eye specialists resort to Since the retina is lavishly populated with veins, strange spillage of "Vitrectomy", a methodology where glassy is taken out from the eye blood from harmed veins there can bring about liquid amassing utilizing little instruments, additionally stripping off scar tissues inside the macula. Diabetic Retinopathy (DR) an eye illness harming the macula because of foothold [5]. -

Foveal Differentiation and Inner Retinal Displacement Are Arrested in Extremely Premature Infants
Retina Foveal Differentiation and Inner Retinal Displacement Are Arrested in Extremely Premature Infants Matthew L. O’Sullivan,1,2 Gui-Shuang Ying,3 Shwetha Mangalesh,1 Vincent Tai,1 Heena R. Divecha,1 Katrina P. Winter,1 Cynthia A. Toth,1 and Xi Chen1; for the BabySTEPS Group 1Department of Ophthalmology, Duke University, Durham, North Carolina, United States 2Ophthalmology Residency Program, Duke University, Durham, North Carolina, United States 3Center for Preventive Ophthalmology and Biostatistics, University of Pennsylvania, Philadelphia, Pennsylvania, United States Correspondence: Xi Chen, PURPOSE. Children with a history of prematurity often have poorly developed foveae but Department of Ophthalmology, when during development foveal differences arise. We hypothesize that the course of Ophthalmology Residency Program, foveal development is altered from the time of preterm birth. Duke University, 2351 Erwin RD, DUMC 3802, Durham, NC 27710, METHODS. Eyes of 102 preterm infants undergoing retinopathy of prematurity screen- USA; ing examinations in the STudy of Eye imaging in Premature infantS (BabySTEPS) [email protected]. (NCT02887157) were serially imaged between 30 and 42 weeks postmenstrual age (PMA) Received: September 4, 2020 using handheld optical coherence tomography systems. Total retinal thickness, inner reti- Accepted: January 27, 2021 nal layer (IRL) thickness, and outer retinal layer (ORL) thickness were measured at the Published: February 18, 2021 foveal center and parafovea. Foveal put depth, IRL thickness, and ORL thickness were compared between infants born at different gestational ages using mixed effects models. Citation: O’Sullivan ML, Ying G-S, Mangalesh S, et al. Foveal RESULTS. Foveal pit depth and IRL thickness were inversely related to gestational age; on differentiation and inner retinal average, the most premature infants had the thickest IRL and shallowest pits at all PMAs. -

Corneal Ectasia Following Laser in Situ Keratomileusis for Myopia
CASE REPORTS AND SMALL CASE SERIES high myopia resulting from poste- results might be related to the pre- Photorefractive Keratectomy rior lenticonus. Postsurgical refrac- existing corneal stromal abnormali- for Correction of tion was stable for 8 years, then a ties in their patients, which were not Epikeratophakia Regression rapid myopic regression of the epi- observed in our group. Thus, PRK keratophakic lenses was observed can effectively be used to treat epi- Excimer laser photorefractive kera- the following year (Table). In- keratophakic regressed lenses in a se- tectomy (PRK) is widely used for the stead of removing the failed epikera- lected group of patients in whom correction of myopia, astigmatism, tophakic lenses, we performed PRK both the epikeratograft and the sur- and hyperopia.1,2 It has also been on the eyes. rounding cornea are clear. This used for correction of astigmatism method eliminates the need for re- after penetrating keratoplasty.3 Results. Two and a half years after moval of the epikeratograft and ex- Epikeratophakia has been used PRK, the refraction in all 4 eyes is posing the patient to the risks of suc- in the treatment of nontolerant con- stable and the epigrafts are clear. The cessive penetrating keratoplasty. tact lens keratoconous patients.4,5 Table presents the refraction and vi- The epigrafts were made from ma- sual acuity results for the eyes be- Hirsh Ami, MD chined corneal tissue that was found fore PRK and at 3 months, 1 year, Solberg Yoram, MD, PhD unsuitable for penetrating kerato- and 21⁄2 years after PRK. No haze has Cahana Michael, MD plasty.