Short Term Presence of Subretinal Fluid in Central Serous Chorioretinopathy Affects Retinal Thickness and Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Cataract Cystoid Macular Oedema Prevention – Update 2019
Review Cystoid Macular Oedema Post-cataract Cystoid Macular Oedema Prevention – Update 2019 Andrzej Grzybowski,1,2 Reda Zemaitiene,3 Lina Mikalauskiene3 1. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland; 2. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland; 3. Department of Ophthalmology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania DOI: https://doi.org/10.17925/EOR.2019.13.1.37 seudophakic cystoid macular oedema (PCMO) is a common complication following both uncomplicated and complicated cataract surgery, becoming apparent about 6 weeks following surgery. PCMO may be asymptomatic in some cases, but in others is associated Pwith a reduction in visual acuity. The pathogenesis of PCMO is linked to postoperative inflammation and the release of inflammatory mediators. The use of topical steroids and/or nonsteroidal anti-inflammatory drugs can reduce the adverse effects of inflammation and have been used for the prevention and treatment of PCMO. However, the therapeutic effectiveness of these drugs is currently not well understood, partially because PCMO can spontaneously resolve as well as the multiple treatment protocols and the paucity of robust data exist. In this review we compare the various prophylactic options for PCMO and provide commentary on their efficacy. Keywords Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been Pseudophakic cystoid macular oedema, offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing post-operative inflammation, cataract surgery, postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract nonsteroidal anti-inflammatory drugs surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in Disclosure: Andrzej Grzybowski, Reda inclusion criteria, patient characteristics, prescription and duration of treatment. -

WSPOS Worldwide Webinar 16: Amblyopia - How and When
Answers to Audience Questions - WSPOS Worldwide Webinar 16: Amblyopia - How and When WWW 16 Panellists Anna Horwood Celeste Mansilla David Granet Krista Kelly Lionel Kowal Susan Cotter Yair Morad Anna Horwood (AH), Celeste Mansilla (CM), Krista Kelly (KK), Lionel Kowal (LK), Susan Cotter (SC), Yair Morad (YM) 1. How do you maintain attained iso visual acuity after successful amblyopia treatment? AH: Intermittent monitoring. If they have regressed previously, I might carry on very intermittent occlusion (an hour or two a week) until I was sure it was stable. CM: With gradual and controlled reduction of the treatment, for example: if the patient had 1 hour of patch per day, I leave it with 1 hour 3 times a week during a month. I do a check and if the visual acuity was maintained, I lower patches to 2 times a week. I keep checking and going down like this until I suspend the treatment. If at any time I detect worsening visual acuity, I return to the previous treatment. SC: Best way is attainment of normal binocular vision. I do not worry about ansiometropic amblyopes who have random dot stereopsis post-treatment. If have constant unilateral strabismus, I can do some limited part-time patching, decreasing patching dosage over time given no regression of VA. YM: repeat examination every 6 months. If I see regression, I will prescribe patching for 30 min a day. 2. How do you plan for very dense amblyopes? AH: I very rarely see them because, with screening, they are picked up early and usually do well. -

The Usefulness of Systemic Inflammatory Markers As Diagnostic
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE The usefulness of systemic inflammatory markers as diagnostic indicators of the pathogenesis of diabetic macular edema A utilidade de marcadores inflamatórios sistêmicos como indicadores diagnósticos da patogênese do edema macular diabético Cagri Ilhan1 , Mehmet Citirik2, Mehmet Murat Uzel3, Hasan Kiziltoprak4, Kemal Tekin5 1. Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey. 2. Department of Ophthalmology, University of Health Sciences, Ankara Ulucanlar Eye Education and Research Hospital, Ankara, Turkey. 3. Department of Ophthalmology, Balikesir University, Balikesir, Turkey. 4. Department of Ophthalmology, Bingol Maternity and Child Hospital, Bingol, Turkey. 5. Department of Ophthalmology, Ercis State Hospital, Van, Turkey. ABSTRACT | Purpose: To investigate the usefulness of sys- groups were similar (diabetic macular edema vs. non-diabetic temic inflammatory markers [i.e., white blood cell and platelet macular edema, p=0.08; diabetic macular edema vs. control, counts, mean platelet volume, and their ratios] as diagnostic p=0.02; and non- diabetic macular edema vs. control, p=0.78). markers of the pathogenesis of diabetic macular edema. Me- All other parameters were similar between groups (all p>0.05). thods: The study cohort included 80 diabetic macular edema Conclusion: The neutrophil/lymphocyte ratio and mean platelet patients (40 with diabetic retinopathy and 40 without) and 40 volume of the diabetic macular edema group were higher than healthy age- and sex-matched controls. Neutrophil, lymphocyte, those of the non-diabetic macular edema and control groups. monocyte, and platelet counts, and the mean platelet volume A neutrophil/lymphocyte ratio cutoff value of ≥2.26 was identified were determined from peripheral blood samples, and the as an indicator of the pathogenesis of diabetic macular edema monocyte/lymphocyte, platelet/lymphocyte, and mean platelet with high sensitivity and specificity. -

Strabismus, Amblyopia & Leukocoria
Strabismus, Amblyopia & Leukocoria [ Color index: Important | Notes: F1, F2 | Extra ] EDITING FILE Objectives: ➢ Not given. Done by: Jwaher Alharbi, Farrah Mendoza. Revised by: Rawan Aldhuwayhi Resources: Slides + Notes + 434 team. NOTE: F1& F2 doctors are different, the doctor who gave F2 said she is in the exam committee so focus on her notes Amblyopia ● Definition Decrease in visual acuity of one eye without the presence of an organic cause that explains that decrease in visual acuity. He never complaints of anything and his family never noticed any abnormalities ● Incidence The most common cause of visual loss under 20 years of life (2-4% of the general population) ● How? Cortical ignorance of one eye. This will end up having a lazy eye ● binocular vision It is achieved by the use of the two eyes together so that separate and slightly dissimilar images arising in each eye are appreciated as a single image by the process of fusion. It’s importance 1. Stereopsis 2. Larger field If there is no coordination between the two eyes the person will have double vision and confusion so as a compensatory mechanism for double vision the brain will cause suppression. The visual pathway is a plastic system that continues to develop during childhood until around 6-9 years of age. During this time, the wiring between the retina and visual cortex is still developing. Any visual problem during this critical period, such as a refractive error or strabismus can mess up this developmental wiring, resulting in permanent visual loss that can't be fixed by any corrective means when they are older Why fusion may fail ? 1. -

Amblyopia HANDOUT ACES
AMBLYOPIA CORNEA PUPIL CATARACT IRIS LENS RETINA MACULA OPTIC NERVE The eye on the right is at risk for all three types of AMBLYOPIA. Rays of light enter the normal eye on the left, are bent by the cornea and the lens and are focused one the most precise part of the retina called the macula. Light entering the right eye is disrupted by a congenital cataract (deprivational amblyopia). Since the right eye is shorter than the left, light doesn't focus on the retina due to unequal far-sightedness(refractive amblyopia). Since the left eye is crossed (esotropia-type strabismus), incoming light fails to align on the macula (strabismic amblyopia). ye doctors and orthoptists want each child to grow frequently suppresses or "turns off" the brain image from up with the healthiest visual system possible. the non-dominant eye. Strabismic amblyopia can be E This goal requires the close cooperation of treated by combinations of drops, glasses, patching parents, pediatricians, primary doctors, optometrists, and/or eye muscle surgery. school nurses and health aids and the professionals who DETECTION: Within the first days after birth, deal with visually impaired babies. part of each baby's first physical exam is the "red reflex" an abnormality of which could indicate cataract or tumor. At birth, a normal infant has relatively poor vision in the range A part of routine pre-school pediatric check-ups is of 20/2000! Under normal conditions, the visual system improves so that observations of red reflex by photoscreen and Brückner 20/20 vision might be attained by school age and retained after age 10 years. -

Optical Coherence Tomography Demonstrates Subretinal Macular Edema from Papilledema
CLINICAL SCIENCES Optical Coherence Tomography Demonstrates Subretinal Macular Edema From Papilledema Vincent J. Hoye III, MD; Audina M. Berrocal, MD; Thomas R. Hedges III, MD; Maria Luz Amaro-Quireza, OD Objective: To evaluate macular changes in eyes with duction in visual acuity. The subretinal fluid appeared papilledema from increased intracranial pressure using to arise from the peripapillary region, and all showed optical coherence tomography (OCT). some improvement in central vision as the fluid re- solved. Methods: Fifty-five patients with papilledema seen dur- ing 1998 and 1999 were studied with OCT of the optic Conclusions: Subretinal fluid accumulations can cause nerve and retinal nerve fiber layer. Nineteen of these also decreased visual acuity in patients with papilledema. Op- had OCT of the macula during periods of acute, sub- tical coherence tomography can demonstrate subretinal acute, or recurrent papilledema and were evaluated in fluid and can be used to follow the course of this impor- detail for this report. tant visual complication of papilledema. Results: Seven patients had OCT evidence of sub- retinal fluid involving the macula. All had some re- Arch Ophthalmol. 2001;119:1287-1290 OSS OF visual acuity from pap- mal findings on neuroimaging scans and illedema or optic nerve head elevated opening pressures on lumbar swelling due to increased in- puncture. Two patients had brain metas- tracranial pressure is uncom- tases, one from breast cancer and the other mon. The visual conse- from testicular cancer. Six patients were Lquences of papilledema usually are limited treated medically with acetazolamide to visual field defects and enlargement of and/or furosemide. -

Human Amblyopia
Human Amblyopia • “Lazy Eye” • Relatively common developmental visual disorder (~2%) • Reduced visual acuity in an otherwise healthy and properly corrected eye • Associated with interruption of normal early visual experience • Most common cause of vision loss in children • Well characterized behaviorally, not neurologically • Treated by patching in children Visual Deficits in Amblyopia • Reduced monoc. visual acuity - defining feature – Usually 20/30 - 20/60 • Impaired contrast sensitivity – Prominent at high spatial frequencies Contrast Sensitivity Sensitivity Contrast – Central visual field is generally most affected Spatial Frequency • Moderate deficits in object segmentation/recognition and spatial localization • Severe deficits in binocular interactions Subtypes of Amblyopia • Anisometropic – Unequal refractive error between the two eyes • Strabismic – Deviated eye that may or may not have unbalanced refraction • Deprivation – Congenital cataract; corneal opacity; eyelid masses Mechanisms of Amblyopia 1. Form deprivation . Sharp image is not formed at the retina 2. Abnormal binocular vision . Binocularity is often changed or lost in amblyopia Models of Amblyopia • Competition hypothesis originated with experiments in kittens in the 1960s by Hubel and Wiesel • Monocular deprivation of retinal input during ‘critical’ developmental periods leads to striking abnormalities in the physiology of visual cortical neurons • Binocular deprivation actually leads to less severe abnormalities • Amblyopia may be a form of activity-dependent deprivation, -
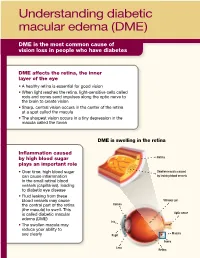
Understanding Diabetic Macular Edema (DME)
Understanding diabetic macular edema (DME) DME is the most common cause of vision loss in people who have diabetes DME affects the retina, the inner layer of the eye • A healthy retina is essential for good vision • When light reaches the retina, light-sensitive cells called rods and cones send impulses along the optic nerve to the brain to create vision • Sharp, central vision occurs in the center of the retina at a spot called the macula • The sharpest vision occurs in a tiny depression in the macula called the fovea DME is swelling in the retina Inflammation caused by high blood sugar Retina plays an important role • Over time, high blood sugar Swollen macula caused can cause inflammation by leaking blood vessels in the small retinal blood vessels (capillaries), leading to diabetic eye disease • Fluid leaking from these blood vessels may cause Vitreous gel Cornea the central part of the retina (the macula) to swell. This Optic nerve is called diabetic macular edema (DME) Iris • The swollen macula may reduce your ability to Macula see clearly Pupil Fovea Lens Retina Understanding DME (continued) Symptoms of DME • Early DME may not have any symptoms • As DME worsens, it can cause blurry central vision ranging from mild to severe • DME can cause significant vision loss over time How DME can affect vision Mild blurriness Moderate blurriness Severe blurriness Untreated DME can lead to vision loss • Talk to your doctor about treatment options • If you develop cataracts (hazy spots that can affect vision) in the lenses of your eyes, the natural lenses may be surgically removed and replaced with artificial ones • Having cataracts or artificial lenses can affect your treatment options ©2014 Allergan, Inc., Irvine, CA 92612 APC67SH14 141256. -

Eye Care for EB Patients Debra.Org
Eye Care for EB Patients Strategies to prevent blistering, scarring and vision loss DEBRA Care Conference 7.23.18 Vicki M. Chen, MD Assistant Professor of Ophthalmology New England Eye Center Tufts Medical Center / Floating Hospital for Children Boston, MA Financial Disclosures • None relevant Lecture Outline 1. What EB related problems can occur in the eye? 2. How can we prevent these problems? 3. Can we do more to reduce pain and vision loss? 4. Is research for EB related eye problems moving forward? What EB related problems can occur in the eye? • The most common problem is corneal abrasion • Cause is: dryness, injury, blister, erosion Missing epithelium… Infection Standard of care is to see patients every 2-3 days until abrasion is healed Why do abrasions occur in EB? • The surface of the eye is similar to skin • It has collagen VII and laminin-332 (5) which form an anchoring complex Corneal basement membrane ...similar to skin What other problems can occur? • Infected abrasion = ulcer • Scarring is common • Severe scars are white and block vision Infection Mild scar Severe scar Astigmatism can lead to amblyopia • Astigmatism causes distortion of images • In young children (under 10 years) astigmatism causes amblyopia • Amblyopia: poor vision development, can be permanent Left eye Right eye Vision does not develop in the eye with high astigmatism = Amblyopia Another common problem is blepharitis OIL NO OIL • Scarring closes oil glands, causes dryness • Dry eyes are more likely to erode • Inflammation due to mild bacterial infection • BKC: severe dryness causes corneal scarring and abnormal blood vessels to grow (seen in non-EB patients) Other eye problems… Tear drainage system bands of conjunctiva (symblepharon) watery eyes from clogged tear duct (obstruction) When do these problems start? JEB H + nH RDEB-HS • Typically JEB and RDEB patients are at higher risk for eye problems • Some start as early as 4-6 months of age • 30% of JEB and 10% of RDEB patients scar within first 10 years which is the critical time of vision development Graph from: J.D. -

Keratoconus Into Focus
SEPTEMBER 2019 # 37 In My View In Practice Profession Sitting Down With Musings of a prospective The amblyopia app making Why the fight for female Stefanie Schmickler: business- glaucoma patient screening accessible to all leadership is far from over minded, patient-focused 12 – 13 32 – 35 46 – 49 50 – 51 Bringing Keratoconus into Focus Sharpening up our response to this underdiagnosed condition 14– 26 NORTH AMERICA www.theophthalmologist.com FOR ROTATIONAL STABILITY, THERE’S NO COMPARISON1,2 1. Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;0:1-7. 2. Potvin R, et al. Toric intraoclar lens orientation and residual refractive astigmatism: an analysis. Clin Ophthalmol. 2016;10:1829-1836. Please see Important Product Information on the adjacent page. AcrySof®IQ Toric ASTIGMATISM-CORRECTING IOL © 2018 Novartis 7/18 US-TOR-18-E-1605 105064 US-TOR-18-E-1605 TO.indd 1 1/30/19 4:04 PM ACRYSOF® IQ TORIC IOL IMPORTANT PRODUCT INFORMATION CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician. INDICATIONS: The AcrySof® IQ Toric posterior chamber intraocular lenses are Image intended for primary implantation in the capsular bag of the eye for visual correction of aphakia and pre-existing corneal astigmatism secondary to removal of a cataractous lens in of the adult patients with or without presbyopia, who desire improved uncorrected distance vision, reduction of residual refractive cylinder and Month increased spectacle independence for distance vision. WARNING/PRECAUTION: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. -

Presbyopia, Anisometropia, and Unilateral Amblyopia
REFRACTIVE SURGERY COMPLEX CASE MANAGEMENT Section Editors: Karl G. Stonecipher, MD; Parag A. Majmudar, MD; and Stephen Coleman, MD Presbyopia, Anisometropia, and Unilateral Amblyopia BY MITCHELL A. JACKSON, MD; LOUIS E. PROBST, MD; AND JONATHAN H. TALAMO, MD CASE PRESENTATION A 45-year-old female nurse is interested in LASIK. She the AMO WaveScan WaveFront System (Abbott Medical does not wear glasses. She says there has always been a large Optics Inc.) for the patient’s right eye calculated a pre- difference in prescription between her eyes and that she scription at 4 mm of +2.20 -4.22 X 9 across a 5.75-mm rarely wore glasses in the past. Her UCVA is 20/200 OD, cor- pupillary diameter. This had a corresponding higher-order recting to 20/30 with +1.75 -4.25 X 180. Her UCVA is 20/40 aberration root mean square error of 0.20 µm. The OS, correcting to 20/15 with -0.75 -0.25 X 30. The patient is patient’s left eye had a calculated prescription of -0.56 right-handed, and her left eye is dominant. Central ultra- -0.21 X 31, also at 4 mm. The pupillary diameter of her left sound pachymetry measures 488 µm OD and 480 µm OS. eye was 5.25 mm, and the higher-order aberration root Figure 1 shows TMS4 (Tomey Corp.) topography of the mean square error was determined to be 0.17 µm patient’s right and left eyes, respectively. Both the (Figure 2). Klyce/Maeda and Smolek/Klyce Keratoconus Screening How would you counsel this patient regarding her suitability Systems are highlighted. -

Strabismus, Amblyopia Management and Leukocoria 431Team
Strabismus, Amblyopia Management and Leukocoria Done By: Tareq Mahmoud Aljurf Lecture mostly contains pictures, but the doctor gave a lot of additional info which we added here. Leukocoria Leukocoria is white opacity of the pupil, and it is a sign not a diagnosis. Causes will be presented going backwards through the eye structures: 1. Cataract Cataract: can be congenital or acquired, usually causes blurred vision and glare. Using the ophthalmoscope if you see nice red reflex on both eyes (pic on right.) unlikely to have any visual problems. Doctor’s notes: Congenital cataract is very important, because if you don’t treat it in the first months of life Irreversible amblyopia. For the brain to unify the 2 images both should have the same shape, size and clarity. If one is clear and the other is not brain gets confused can’t put them together suppresses image from the cataract eye. If this continues for 2,3 or 4 months amblyopia. For example: If the child presents with the problem at 1 year of age already too late, you can’t do anything. (Because amblyopia happens much earlier than 1yr) The eye is connected to the brain Retina and optic nerve regarded as parts of the CNS it’s a neurological problem difficult to reverse after 3 months of suppression. 2. Persistent hyperplastic primary vitreous PHPV is a congenital condition caused by failure of the normal regression of the primary vitreous. It is usually associated with unilateral vision loss Doctor’s notes: During embryology, blood vessels come from the optic nerve to nourish the lens, they usually disappear clear vitreous.