Screening for Visual Impairment in Children Ages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
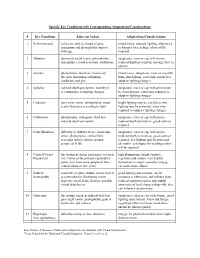
Specific Eye Conditions with Corresponding Adaptations/Considerations
Specific Eye Conditions with Corresponding Adaptations/Considerations # Eye Condition Effect on Vision Adaptations/Considerations 1 Achromotopsia colors are seen as shades of grey, tinted lenses, reduced lighting, alternative nystagmus and photophobia improve techniques for teaching colors will be with age required 2 Albinism decreased visual acuity, photophobia, sunglasses, visor or cap with a brim, nystagmus, central scotomas, strabismus reduced depth perception, moving close to objects 3 Aniridia photophobia, field loss, vision may tinted lenses, sunglasses, visor or cap with fluctuate depending on lighting brim, dim lighting, extra time required to conditions and glare adapt to lighting changes 4 Aphakia reduced depth perception, inability to sunglasses, visor or cap with a brim may accommodate to lighting changes be worn indoors, extra time required to adapt to lighting changes 5 Cataracts poor color vision, photophobia, visual bright lighting may be a problem, low acuity fluctuates according to light lighting may be preferred, extra time required to adapt to lighting changes 6 Colobomas photophobia, nystagmus, field loss, sunglasses, visor or cap with a brim, reduced depth perception reduced depth perception, good contrast required 7 Color Blindness difficulty or inability to see colors and sunglasses, visor or cap with a brim, detail, photophobia, central field reduced depth perception, good contrast scotomas (spotty vision), normal required, low lighting may be preferred, peripheral fields alternative techniques for teaching colors -

A Patient & Parent Guide to Strabismus Surgery
A Patient & Parent Guide to Strabismus Surgery By George R. Beauchamp, M.D. Paul R. Mitchell, M.D. Table of Contents: Part I: Background Information 1. Basic Anatomy and Functions of the Extra-ocular Muscles 2. What is Strabismus? 3. What Causes Strabismus? 4. What are the Signs and Symptoms of Strabismus? 5. Why is Strabismus Surgery Performed? Part II: Making a Decision 6. What are the Options in Strabismus Treatment? 7. The Preoperative Consultation 8. Choosing Your Surgeon 9. Risks, Benefits, Limitations and Alternatives to Surgery 10. How is Strabismus Surgery Performed? 11. Timing of Surgery Part III: What to Expect Around the Time of Surgery 12. Before Surgery 13. During Surgery 14. After Surgery 15. What are the Potential Complications? 16. Myths About Strabismus Surgery Part IV: Additional Matters to Consider 17. About Children and Strabismus Surgery 18. About Adults and Strabismus Surgery 19. Why if May be Important to a Person to Have Strabismus Surgery (and How Much) Part V: A Parent’s Perspective on Strabismus Surgery 20. My Son’s Diagnosis and Treatment 21. Growing Up with Strabismus 22. Increasing Signs that Surgery Was Needed 23. Making the Decision to Proceed with Surgery 24. Explaining Eye Surgery to My Son 25. After Surgery Appendix Part I: Background Information Chapter 1: Basic Anatomy and Actions of the Extra-ocular Muscles The muscles that move the eye are called the extra-ocular muscles. There are six of them on each eye. They work together in pairs—complementary (or yoke) muscles pulling the eyes in the same direction(s), and opposites (or antagonists) pulling the eyes in opposite directions. -

Visual Performance of Scleral Lenses and Their Impact on Quality of Life In
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE Visual performance of scleral lenses and their impact on quality of life in patients with irregular corneas Desempenho visual das lentes esclerais e seu impacto na qualidade de vida de pacientes com córneas irregulares Dilay Ozek1, Ozlem Evren Kemer1, Pinar Altiaylik2 1. Department of Ophthalmology, Ankara Numune Education and Research Hospital, Ankara, Turkey. 2. Department of Ophthalmology, Ufuk University Faculty of Medicine, Ankara, Turkey. ABSTRACT | Purpose: We aimed to evaluate the visual quality CCS with scleral contact lenses were 0.97 ± 0.12 (0.30-1.65), 1.16 performance of scleral contact lenses in patients with kerato- ± 0.51 (0.30-1.80), and 1.51 ± 0.25 (0.90-1.80), respectively. conus, pellucid marginal degeneration, and post-keratoplasty Significantly higher contrast sensitivity levels were recorded astigmatism, and their impact on quality of life. Methods: with scleral contact lenses compared with those recorded with We included 40 patients (58 eyes) with keratoconus, pellucid uncorrected contrast sensitivity and spectacle-corrected contrast marginal degeneration, and post-keratoplasty astigmatism who sensitivity (p<0.05). We found the National Eye Institute Visual were examined between October 2014 and June 2017 and Functioning Questionnaire overall score for patients with scleral fitted with scleral contact lenses in this study. Before fitting contact lens treatment to be significantly higher compared with scleral contact lenses, we noted refraction, uncorrected dis- that for patients with uncorrected sight (p<0.05). Conclusion: tance visual acuity, spectacle-corrected distance visual acuity, Scleral contact lenses are an effective alternative visual correction uncorrected contrast sensitivity, and spectacle-corrected contrast method for keratoconus, pellucid marginal degeneration, and sensitivity. -

WSPOS Worldwide Webinar 16: Amblyopia - How and When
Answers to Audience Questions - WSPOS Worldwide Webinar 16: Amblyopia - How and When WWW 16 Panellists Anna Horwood Celeste Mansilla David Granet Krista Kelly Lionel Kowal Susan Cotter Yair Morad Anna Horwood (AH), Celeste Mansilla (CM), Krista Kelly (KK), Lionel Kowal (LK), Susan Cotter (SC), Yair Morad (YM) 1. How do you maintain attained iso visual acuity after successful amblyopia treatment? AH: Intermittent monitoring. If they have regressed previously, I might carry on very intermittent occlusion (an hour or two a week) until I was sure it was stable. CM: With gradual and controlled reduction of the treatment, for example: if the patient had 1 hour of patch per day, I leave it with 1 hour 3 times a week during a month. I do a check and if the visual acuity was maintained, I lower patches to 2 times a week. I keep checking and going down like this until I suspend the treatment. If at any time I detect worsening visual acuity, I return to the previous treatment. SC: Best way is attainment of normal binocular vision. I do not worry about ansiometropic amblyopes who have random dot stereopsis post-treatment. If have constant unilateral strabismus, I can do some limited part-time patching, decreasing patching dosage over time given no regression of VA. YM: repeat examination every 6 months. If I see regression, I will prescribe patching for 30 min a day. 2. How do you plan for very dense amblyopes? AH: I very rarely see them because, with screening, they are picked up early and usually do well. -

Fact Sheet: Refractive Errors
Fact Sheet: Refractive Errors More than 11 million Americans have common vision problems that can be corrected with the use of prescriptive eyewear such as glasses or contact lenses.1 These conditions are known as refractive errors and they occur when the eye doesn’t correctly bend, or ―refract,‖ light as it enters the eye. Common refractive errors include the following: o Nearsightedness (also called myopia)—A condition where objects up close appear clearly, while objects far away appear blurry. With nearsightedness, light comes to focus in front of the retina instead of on the retina. o Farsightedness (also called hyperopia)—A common type of refractive error where distant objects may be seen more clearly than objects that are near. However, people experience farsightedness differently. Some people may not notice any problems with their vision, especially when they are young. For people with significant farsightedness, vision can be blurry for objects at any distance, near or far. o Astigmatism—A condition in which the eye does not focus light evenly onto the retina, the light-sensitive tissue at the back of the eye. This can cause images to appear blurry and stretched out. o Presbyopia—An age-related condition in which the ability to focus up close becomes more difficult. As the eye ages, the lens can no longer change shape enough to allow the eye to focus close objects clearly. Refractive errors are one of the most common—and correctable—causes of visual impairment in the United States. According to a recent study led by the National Eye Institute (NEI), approximately half of all American adults don’t have the 20/20 vision physicians consider optimal due to refractive errors.2 Women experience refractive error more frequently than men: Twenty-six percent more women aged 12 and older have uncorrected visual impairment due to refractive error compared with men aged 12 and older. -

Hyperopia Hyperopia
Hyperopia Hyperopia hyperopia hyperopia • Farsightedness, or hyperopia, • Farsightedness occurs if your eyeball is too as it is medically termed, is a short or the cornea has too little curvature, so vision condition in which distant objects are usually light entering your eye is not focused correctly. seen clearly, but close ones do • Its effect varies greatly, depending on the not come into proper focus. magnitude of hyperopia, the age of the individual, • Approximately 25% of the the status of the accommodative and general population is hyperopic (a person having hyperopia). convergence system, and the demands placed on the visual system. By Judith Lee and Gretchyn Bailey; reviewed by Dr. Vance Thompson; Flash illustration by Stephen Bagi 1. Cornea is too flap. hyperopia • In theory, hyperopia is the inability to focus and see the close objects clearly, but in practice many young hyperopics can compensate the weakness of their focusing ability by excessive use of the accommodation functions of their eyes. Hyperopia is a refractive error in • But older hyperopics are not as lucky as them. By which parallel rays of light aging, accommodation range diminishes and for 2. Axial is too short. entering the eye reach a focal older hyperopics seeing close objects becomes point behind the plane of the retina, while accommodation an impossible mission. is maintained in a state of relaxation. 1 Amplitude of Accommodation hyperopia Maximum Amplitude= 25-0.4(age) • An emmetropic eye for reading and other near Probable Amplitude= 18.5-.3(age) work, at distance of 16 in (40cm), the required amount of acc. -

Surgical Options in Managing Convergent Strabismus Fixus Related to High Myopia
Ophthalmol Ina 2015;41(2):107-115 107 Case Report Surgical Options in Managing Convergent Strabismus Fixus Related to High Myopia Jessica Zarwan, Gusti G Suardana, Anna P Bani Department of Ophthalmology, Faculty of Medicine, Indonesia University Cipto Mangunkusumo Hospital, Jakarta ABSTRACT Background: Convergent strabismus fixus is a condition in which one or both eyes are anchored in extreme adduction. This condition is caused by superotemporal herniation of the enlarged globe from the muscle cone through the space between the superior and lateral rectus muscle. It is commonly related to high myopia. Recently, transposition type procedure which unite the superior and lateral rectus muscles were proposed as the treatment of choice in this condition. However, there is no clear consensus regarding the best surgical procedure in convergent strabismus fixus. This case series reported three rare cases of convergent strabismus fixus related to high myopia and highlight the option of surgical procedure that can be done in these cases. Case Illustration: Three patients presented with convergent strabismus fixus of both eyes with history of bilateral high myopia since childhood. All patients showed Krimsky tests of >95∆ET preoperatively, with medial displacement of superior rectus and inferior displacement of lateral rectus of both eyes in all patients on orbital imaging. The bilateral Yokoma procedure both resulted in an orthophoric postoperative result with marked improvement in abduction and elevation. The hemi-Jensen also showed a significant improvement of over than 60∆ on the operated eye, resorting it to a central position during primary gaze. Conclusion: Convergent strabismus fixus is a rare condition and frequently associated with high myopia. -

Strabismus, Amblyopia & Leukocoria
Strabismus, Amblyopia & Leukocoria [ Color index: Important | Notes: F1, F2 | Extra ] EDITING FILE Objectives: ➢ Not given. Done by: Jwaher Alharbi, Farrah Mendoza. Revised by: Rawan Aldhuwayhi Resources: Slides + Notes + 434 team. NOTE: F1& F2 doctors are different, the doctor who gave F2 said she is in the exam committee so focus on her notes Amblyopia ● Definition Decrease in visual acuity of one eye without the presence of an organic cause that explains that decrease in visual acuity. He never complaints of anything and his family never noticed any abnormalities ● Incidence The most common cause of visual loss under 20 years of life (2-4% of the general population) ● How? Cortical ignorance of one eye. This will end up having a lazy eye ● binocular vision It is achieved by the use of the two eyes together so that separate and slightly dissimilar images arising in each eye are appreciated as a single image by the process of fusion. It’s importance 1. Stereopsis 2. Larger field If there is no coordination between the two eyes the person will have double vision and confusion so as a compensatory mechanism for double vision the brain will cause suppression. The visual pathway is a plastic system that continues to develop during childhood until around 6-9 years of age. During this time, the wiring between the retina and visual cortex is still developing. Any visual problem during this critical period, such as a refractive error or strabismus can mess up this developmental wiring, resulting in permanent visual loss that can't be fixed by any corrective means when they are older Why fusion may fail ? 1. -

Management of Vith Nerve Palsy-Avoiding Unnecessary Surgery
MANAGEMENT OF VITH NERVE PALSY-AVOIDING UNNECESSARY SURGERY P. RIORDAN-E VA and J. P. LEE London SUMMARY for unrecovered VIth nerve palsy must involve a trans Unresolved Vlth nerve palsy that is not adequately con position procedure3.4. The availability of botulinum toxin trolled by an abnormal head posture or prisms can be to overcome the contracture of the ipsilateral medial rectus 5 very suitably treated by surgery. It is however essential to now allows for full tendon transplantation techniques -7, differentiate partially recovered palsies, which are with the potential for greatly increased improvements in amenable to horizontal rectus surgery, from unrecovered final fields of binocular single vision, and deferment of palsies, which must be treated initially by a vertical any necessary surgery to the medial recti, which is also muscle transposition procedure. Botulinum toxin is a likely to improve the final outcome. valuable tool in making this distinction. It also facilitates This study provides definite evidence, from a large full tendon transposition in unrecovered palsies, which series of patients, of the potential functional outcome from appears to produce the best functional outcome of all the the surgical treatment of unresolved VIth nerve palsy, transposition procedures, with a reduction in the need for together with clear guidance as to the forms of surgery that further surgery. A study of the surgical management of 12 should be undertaken in specific cases. The fundamental patients with partially recovered Vlth nerve palsy and 59 role of botulinum toxin in establishing the degree of lateral patients with unrecovered palsy provides clear guidelines rectus function and hence the correct choice of initial sur on how to attain a successful functional outcome with the gery, and as an adjunct to transposition surgery for unre minimum amount of surgery. -

Associations Between Hyperopia and Other Vision and Refractive Error Characteristics
1040-5488/14/9104-0383/0 VOL. 91, NO. 4, PP. 383Y389 OPTOMETRY AND VISION SCIENCE Copyright * 2014 American Academy of Optometry ORIGINAL ARTICLE Associations between Hyperopia and Other Vision and Refractive Error Characteristics Marjean Taylor Kulp*, Gui-shuang Ying†, Jiayan Huang‡, Maureen Maguire†, Graham Quinn§, Elise B. Ciner||, Lynn A. Cyert**, Deborah A. Orel-Bixler††, and Bruce D. Moore||, for the VIP Study Group ABSTRACT Purpose. To investigate the association of hyperopia greater than +3.25 diopters (D) with amblyopia, strabismus, aniso- metropia, astigmatism, and reduced stereoacuity in preschoolers. Methods. Three- to five-year-old Head Start preschoolers (N = 4040) underwent vision examination including monocular visual acuity (VA), cover testing, and cycloplegic refraction during the Vision in Preschoolers Study. Visualacuity was tested with habitual correction and was retested with full cycloplegic correction when VAwas reduced below age norms in the presence of significant refractive error. Stereoacuity testing (Stereo Smile II) was performed on 2898 children during study years 2 and 3. Hyperopia was classified into three levels of severity (based on the most positive meridian on cycloplegic refraction): group 1: greater than or equal to +5.00 D, group 2: greater than +3.25 D to less than +5.00 D with interocular difference in spherical equivalent greater than or equal to 0.50 D, and group 3: greater than +3.25 D to less than +5.00 D with interocular difference in spherical equivalent less than 0.50 D. ‘‘Without’’ hyperopia was defined as refractive error of +3.25 D or less in the most positive meridian in both eyes. -

Amblyopia HANDOUT ACES
AMBLYOPIA CORNEA PUPIL CATARACT IRIS LENS RETINA MACULA OPTIC NERVE The eye on the right is at risk for all three types of AMBLYOPIA. Rays of light enter the normal eye on the left, are bent by the cornea and the lens and are focused one the most precise part of the retina called the macula. Light entering the right eye is disrupted by a congenital cataract (deprivational amblyopia). Since the right eye is shorter than the left, light doesn't focus on the retina due to unequal far-sightedness(refractive amblyopia). Since the left eye is crossed (esotropia-type strabismus), incoming light fails to align on the macula (strabismic amblyopia). ye doctors and orthoptists want each child to grow frequently suppresses or "turns off" the brain image from up with the healthiest visual system possible. the non-dominant eye. Strabismic amblyopia can be E This goal requires the close cooperation of treated by combinations of drops, glasses, patching parents, pediatricians, primary doctors, optometrists, and/or eye muscle surgery. school nurses and health aids and the professionals who DETECTION: Within the first days after birth, deal with visually impaired babies. part of each baby's first physical exam is the "red reflex" an abnormality of which could indicate cataract or tumor. At birth, a normal infant has relatively poor vision in the range A part of routine pre-school pediatric check-ups is of 20/2000! Under normal conditions, the visual system improves so that observations of red reflex by photoscreen and Brückner 20/20 vision might be attained by school age and retained after age 10 years. -

Visual Impairment 101: What Do Administrators Need to Know?
Visual Impairment 101: What do Administrators Need to Know? Stephanie Walker, ESC Region 11 Mary Shore, TSBVI Outreach Visual Impairment 101 What do administrators need to know? Presented by: Stephanie Walker, M.Ed. Lead for the State Leadership Services for the Blind and Visually Impaired (SLSBVI) Network Mary Shore, COMS Professional Preperation Coordinator TSBVI Outreach Handouts and Additional Resources Texas School for the Blind and Visually Impaired - Administrator’s Toolbox Statewide Leadership Services for the Blind and Visually Impaired Simulation Activities What is a visual impairment? §300.8 (c)(13) Visual impairment including blindness means an impairment in vision that, even with correction, adversely affects a child’s educational performance. The term includes both partial sight and blindness. §89.1040 (c)(12) (A) A student with a visual impairment is one who has been determined to meet the criteria for visual impairment as stated in 34 CFR, §300.8(c)(13). The visual loss should be stated in exact measures of visual field and corrected visual acuity at a distance and at close range in each eye in a report by a licensed ophthalmologist or optometrist. The report should also include prognosis whenever possible. If exact measures cannot be obtained, the eye specialist must so state and provide best estimates. What types of vision does this include? Legally Blind Central vision acuity in the better eye of 20/200 or worse after the best correction possible, or a field of vision of 20 degrees or less. Low Vision/Partial sight Visual acuity better than 20/200 after correction in the better eye.