Paraneoplastic Retinopathy Associated with Metastatic Cutaneous Melanoma of Unknown Primary Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Organization of the Inner Nuclear Layer of the Rabbit Retina
The Journal of Neuroscience. January 1995. 15(l): 875-888 The Organization of the Inner Nuclear Layer of the Rabbit Retina Enrica Strettoil and Richard H. Masland2 ‘Istituto di Neurofisiologia del C.N.R., Pisa, Italy; 2Program in Neuroscience and Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts The initial goal of this study was to establish an accounting function (reviewed by Kolb et al., 198 1; Masland, 1988; Cohen of the major classes of cells present in the inner nuclear and Sterling, 1990; WHssle and Boycott, 199 1; Masland, 1992). layer (INL) of the rabbit’s retina. Series of 80-100 radial What one does not know is how many more cell types there sections 1 pm thick were cut from retinal blocks dissected are-how many cells have not yet been encountered by current, at intervals along the vertical meridian. They were photo- essentially trial and error, methods. graphed at high magnification in the light microscope. By The missing cells-those invisible to present staining tech- visualizing the initial segments of processes leaving the so- niques-are important. They represent unseen actors in the ret- mata, we could identify each cell as a bipolar, amacrine, ina’s circuitry. The responses of the retinal ganglion cells are horizontal, or Muller cell. The identifications made by light controlled by all of the neurons afferent to them. If there are microscopy were confirmed by electron microscopy of al- unseen elements afferent to the ganglion cell, our understanding ternating ultrathin sections. On average, bipolar cells made of the creation of ganglion cell responses risks major error. -

Radial and Tangential Dispersion Patterns in the Mouse Retina Are Cell
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2494-2498, March 1995 Neurobiology Radial and tangential dispersion patterns in the mouse retina are cell-class specific (cell migration/cell lineage/retinal development/transgenic mice/X chromosome inactivation) B. E. REESE*, A. R. HARvEyt, AND S.-S. TANt§ *Neuroscience Research Institute and Department of Psychology, University of California, Santa Barbara, CA 93106; tDepartment of Anatomy and Human Biology, University of Western Australia, Nedlands, WA 6009 Australia; and tDepartment of Anatomy and Cell Biology, University of Melbourne, Parkville, Victoria 3052, Australia Communicated by Pasko Rakic, Yale University School ofMedicine, New Haven, CT, December 16, 1994 ABSTRACT The retina is derived from a pseudostratified retinal cells remain clonally segregated, they should appear as germinal zone in which the relative position of a progenitor distinct groups of blue versus white cells. We have used this cell is believed to determine the position ofthe progeny aligned approach to address the issue of whether radially aligned cells in the radial axis. Such a developmental mechanism would in the mature retina reflect such a clonal derivation. ensure that radial arrays of cells which comprise functional units in the mature central nervous system are also clonally MATERIALS AND METHODS related. The present study has tested this hypothesis by using Retinas from adult transgenic mice, derived from founder line X chromosome-inactivation transgenic mosaic mice. We re- H253, which carries a lacZ transgene -

Quantification of Retinal Layer Thickness Changes in Acute Macular
BJO Online First, published on May 11, 2016 as 10.1136/bjophthalmol-2016-308367 Clinical science Br J Ophthalmol: first published as 10.1136/bjophthalmol-2016-308367 on 11 May 2016. Downloaded from Quantification of retinal layer thickness changes in acute macular neuroretinopathy Marion R Munk,1,2,3 Marco Beck,1 Simone Kolb,1 Michael Larsen,4 Steffen Hamann,4 Christophe Valmaggia,5 Martin S Zinkernagel1,3,6 1Department of ABSTRACT METHODS Ophthalmology, Inselspital, Purpose To quantitatively evaluate retinal layer Patient selection and setting Bern University Hospital, University of Bern, Switzerland thickness changes in acute macular neuroretinopathy This retrospective study included 11 patients from 2Department of (AMN). three tertiary referring institutions: Department of Ophthalmology, Northwestern Methods AMN areas were identified using near- Ophthalmology, Inselspital, Bern University Hospital, University, Feinberg School of infrared reflectance (NIR) images. Intraretinal layer University of Bern, Switzerland; Hospital St Gallen, Medicine, Chicago, Illinois, segmentation using Heidelberg software was performed. St Gallen, Switzerland and Rigshospitalet—Glostrup, USA 3Bern Photographic Reading The inbuilt ETDRS -grid was moved onto the AMN lesion University of Copenhagen, Copenhagen, Denmark. Center, Inselspital, Bern and the mean retinal layer thicknesses of the central grid The study adhered to the tenets of the Declaration of University Hospital, University were recorded and compared with the corresponding Helsinki and was approved by the local review board. of Bern, Switzerland area of the fellow eye at initial presentation and during Retrospectively, patients diagnosed with AMN 4Department of Ophthalmology, Rigshospitalet—Glostrup, follow-up. were included in this study. Diagnosis was based on University of Copenhagen, Results Eleven patients were included (mean age the following clinical criteria: (1) Characteristic Glostrup, Denmark 26±6 years). -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

Primary Retinal Pathology in Multiple Sclerosis As Detected by Optical Coherence Tomography Downloaded From
doi:10.1093/brain/awq346 Brain 2011: 134; 518–533 | 518 BRAIN A JOURNAL OF NEUROLOGY Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography Downloaded from Shiv Saidha,1 Stephanie B. Syc,1 Mohamed A. Ibrahim,2 Christopher Eckstein,1 Christina V. Warner,1 Sheena K. Farrell,1 Jonathan D. Oakley,3 Mary K. Durbin,3 Scott A. Meyer,3 Laura J. Balcer,4 Elliot M. Frohman,5 Jason M. Rosenzweig,1 Scott D. Newsome,1 John brain.oxfordjournals.org N. Ratchford,1 Quan D. Nguyen2 and Peter A. Calabresi1 1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Carl Zeiss Meditec Inc, Dublin, CA, USA 4 Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA 5 Department of Neurology and Ophthalmology, University of Texas Southwestern, Dallas, TX, USA at University of Texas Southwestern Medical Center Dallas on February 23, 2011 Correspondence to: Dr Peter A. Calabresi, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 627, Baltimore, MD 21287, USA E-mail: [email protected] Optical coherence tomography studies in multiple sclerosis have primarily focused on evaluation of the retinal nerve fibre layer. The aetiology of retinal changes in multiple sclerosis is thought to be secondary to optic nerve demyelination. The objective of this study was to use optical coherence tomography to determine if a subset of patients with multiple sclerosis exhibit primary retinal neuronopathy, in the absence of retrograde degeneration of the retinal nerve fibre layer and to ascertain if such patients may have any distinguishing clinical characteristics. -

Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy
Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy Mathias Abegg, MD, PhD,1 Muriel Dysli,1 Sebastian Wolf, MD, PhD,1 Jens Kowal, PhD,2 Pascal Dufour, MSc,2 Martin Zinkernagel, MD, PhD1 Purpose: To investigate retrograde axonal degeneration for its potential to cause microcystic macular edema (MME), a maculopathy that has been previously described in patients with demyelinating disease. To identify risk factors for MME and to expand the anatomic knowledge on MME. Design: Retrospective case series. Participants: We included 117 consecutive patients and 180 eyes with confirmed optic neuropathy of variable etiology. Patients with glaucoma were excluded. Methods: We determined age, sex, visual acuity, etiology of optic neuropathy, and the temporal and spatial characteristics of MME. Eyes with MME were compared with eyes with optic neuropathy alone and to healthy fellow eyes. With retinal layer segmentation we quantitatively measured the intraretinal anatomy. Main Outcome Measures: Demographic data, distribution of MME in the retina, and thickness of retinal layers were analyzed. Results: We found MME in 16 eyes (8.8%) from 9 patients, none of whom had multiple sclerosis or neu- romyelitis optica. The MME was restricted to the inner nuclear layer (INL) and had a characteristic perifoveal circular distribution. Compared with healthy controls, MME was associated with significant thinning of the ganglion cell layer and nerve fiber layer, as well as a thickening of the INL and the deeper retinal layers. Youth is a significant risk factor for MME. Conclusions: Microcystic macular edema is not specific for demyelinating disease. It is a sign of optic neuropathy irrespective of its etiology. -
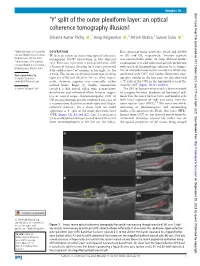
'Y' Split of the Outer Plexiform Layer: an Optical Coherence Tomography
Images in… BMJ Case Rep: first published as 10.1136/bcr-2021-242117 on 8 April 2021. Downloaded from ‘Y’ split of the outer plexiform layer: an optical coherence tomography illusion! Srikanta Kumar Padhy ,1 Anup Kelgaonkar ,2 Ashish Khalsa,2 Suman Sahu 1 1Ophthalmology, LV Prasad Eye DESCRIPTION Best corrected visual acuity was 20/20 and 20/400 Institute Bhubaneswar Campus, We here in report an interesting optical coherence in OD and OS, respectively. Anterior segment Bhubaneswar, Odisha, India was unremarkable under slit lamp. Dilated fundus 2 tomography (OCT) observation in two different Vitreo- Retina, LV Prasad Eye eyes. First case represents a 26- year-old man, with examination revealed subretinal greyish membrane Institute Bhubaneswar Campus, a history of tobacco chewing for 8 years, presented with speck of haemorrhage adjacent to it, sugges- Bhubaneshwar, Odisha, India with sudden onset of scotoma in his right eye for tive of choroidal neovascular membrane which was confirmed with OCT and fundus fluorescein angi- Correspondence to 1 week. The uncorrected visual acuity was 20/40 in Dr Anup Kelgaonkar; right eye (OD) and 20/20 in left eye (OS), respec- ography. Similar to the first case, we also observed anupnk2008@ gmail.com tively. Anterior segment was essentially within a ‘Y’ split of the OPL in the horizontal scan of the normal limits. Right eye fundus examination macular OCT (figure 1B, B1 and B2). Accepted 26 March 2021 revealed a dull foveal reflex with neurosensory The OPL of human retina entails a dense network detachment and subretinal fibrin features sugges- of synapses between dendrites of horizontal cells tive of central serous chorioretinopathy. -
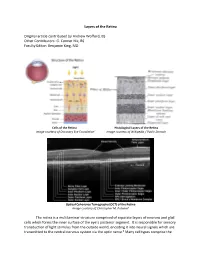
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

Download PDF File
Folia Morphol. Vol. 78, No. 2, pp. 237–258 DOI: 10.5603/FM.a2018.0075 O R I G I N A L A R T I C L E Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Aging changes in the retina of male albino rat: a histological, ultrastructural and immunohistochemical study M.E.I. Mohamed, E.A.A. El-Shaarawy, M.F. Youakim, D.M.A. Shuaib, M.M. Ahmed Department of Anatomy and Embryology, Faculty of Medicine, Cairo University, Cairo, Egypt [Received: 9 June 2018; Accepted: 26 June 2018] Background: Degenerative changes caused by aging may affect the eye, especially the retina. Such changes occur as a part of normal physiological process and may be irreversible. The aim of the study was to demonstrate the influence of aging on the morphology of the retina to provide a basis to explain the pathogenesis of age-associated decline in visual acuity, scotopic and photopic sensitivity. Materials and methods: Forty male albino rats were used and divided into four age groups (group I: age of cortical maturity, group II: middle-aged, group III: aged group and group IV: senile group). The rats were sacrificed, the eye balls were enucleated. Intra-vitreal injections of formalin for haematoxylin and eosin and immunohistochemical sections, glutaraldehyde for toluidine blue semithin and E/M ultra-thin sections were performed. Measurements and quantitative histomorphometric estimation of the layers of the retina were done. Results: Light microscopic examination revealed age-dependent attenuation of photoreceptor striations. Aged and senile groups presented pyknotic, widely- -spaced nuclei of the outer nuclear layer.