'Y' Split of the Outer Plexiform Layer: an Optical Coherence Tomography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantification of Retinal Layer Thickness Changes in Acute Macular
BJO Online First, published on May 11, 2016 as 10.1136/bjophthalmol-2016-308367 Clinical science Br J Ophthalmol: first published as 10.1136/bjophthalmol-2016-308367 on 11 May 2016. Downloaded from Quantification of retinal layer thickness changes in acute macular neuroretinopathy Marion R Munk,1,2,3 Marco Beck,1 Simone Kolb,1 Michael Larsen,4 Steffen Hamann,4 Christophe Valmaggia,5 Martin S Zinkernagel1,3,6 1Department of ABSTRACT METHODS Ophthalmology, Inselspital, Purpose To quantitatively evaluate retinal layer Patient selection and setting Bern University Hospital, University of Bern, Switzerland thickness changes in acute macular neuroretinopathy This retrospective study included 11 patients from 2Department of (AMN). three tertiary referring institutions: Department of Ophthalmology, Northwestern Methods AMN areas were identified using near- Ophthalmology, Inselspital, Bern University Hospital, University, Feinberg School of infrared reflectance (NIR) images. Intraretinal layer University of Bern, Switzerland; Hospital St Gallen, Medicine, Chicago, Illinois, segmentation using Heidelberg software was performed. St Gallen, Switzerland and Rigshospitalet—Glostrup, USA 3Bern Photographic Reading The inbuilt ETDRS -grid was moved onto the AMN lesion University of Copenhagen, Copenhagen, Denmark. Center, Inselspital, Bern and the mean retinal layer thicknesses of the central grid The study adhered to the tenets of the Declaration of University Hospital, University were recorded and compared with the corresponding Helsinki and was approved by the local review board. of Bern, Switzerland area of the fellow eye at initial presentation and during Retrospectively, patients diagnosed with AMN 4Department of Ophthalmology, Rigshospitalet—Glostrup, follow-up. were included in this study. Diagnosis was based on University of Copenhagen, Results Eleven patients were included (mean age the following clinical criteria: (1) Characteristic Glostrup, Denmark 26±6 years). -

Paraneoplastic Retinopathy Associated with Metastatic Cutaneous Melanoma of Unknown Primary Site
PARANEOPLASTIC RETINOPATHY ASSOCIATED WITH METASTATIC CUTANEOUS MELANOMA OF UNKNOWN PRIMARY SITE l 2 l I HA AM KIRATLI , CHARLES E. THIRKILL , SEVGUL BILGI(: , BORA ELDEM YY 1 and ARMAN KE(:ECI Ankara, Turkey and Sacramento, California SUMMARY features of a patient with this rare syndrome are Purpose: To describe further the clinical and immuno described here. logical features of cutaneous melanoma-associated retinopathy, which is an infrequent form of paraneo CASE REPORT plastic syndrome. Methods: We studied the salient clinical and immuno A 66-year-old man without any prior systemic or logical aspects of a 66-year-old man with metastatic ocular problems presented with the complaint of cutaneous melanoma to lymph nodes of unknown mild visual loss of recent onset in his left eye. He had primary site who developed melanoma-associated experienced occasional flashing lights but had no retinopathy. difficulty with night vision. A few days earlier an Results: There was gradual loss of vision in the left eye. incisional biopsy had been done from his right Colour vision and night vision were not affected. Visual axillary region, where rapid enlargement of four or fields showed arcuate defects. A full-field electroretino five lymph nodes each measuring 3 X 2 X 2 cm was gram demonstrated attenuation of the b-wave ampli noticed. tude in the left eye. The a-wave was intact. Indirect His best corrected visual acuity was 6/9 in the right immunofluorescence techniques showed that the anti eye and 6/18 in the left eye. There was no afferent body reactions took place mainly in the outer plexiform pupillary defect. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

Primary Retinal Pathology in Multiple Sclerosis As Detected by Optical Coherence Tomography Downloaded From
doi:10.1093/brain/awq346 Brain 2011: 134; 518–533 | 518 BRAIN A JOURNAL OF NEUROLOGY Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography Downloaded from Shiv Saidha,1 Stephanie B. Syc,1 Mohamed A. Ibrahim,2 Christopher Eckstein,1 Christina V. Warner,1 Sheena K. Farrell,1 Jonathan D. Oakley,3 Mary K. Durbin,3 Scott A. Meyer,3 Laura J. Balcer,4 Elliot M. Frohman,5 Jason M. Rosenzweig,1 Scott D. Newsome,1 John brain.oxfordjournals.org N. Ratchford,1 Quan D. Nguyen2 and Peter A. Calabresi1 1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Carl Zeiss Meditec Inc, Dublin, CA, USA 4 Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA 5 Department of Neurology and Ophthalmology, University of Texas Southwestern, Dallas, TX, USA at University of Texas Southwestern Medical Center Dallas on February 23, 2011 Correspondence to: Dr Peter A. Calabresi, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 627, Baltimore, MD 21287, USA E-mail: [email protected] Optical coherence tomography studies in multiple sclerosis have primarily focused on evaluation of the retinal nerve fibre layer. The aetiology of retinal changes in multiple sclerosis is thought to be secondary to optic nerve demyelination. The objective of this study was to use optical coherence tomography to determine if a subset of patients with multiple sclerosis exhibit primary retinal neuronopathy, in the absence of retrograde degeneration of the retinal nerve fibre layer and to ascertain if such patients may have any distinguishing clinical characteristics. -
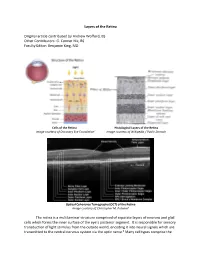
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -
Retinal Layers Handout
Retinal Layers Handout Vasculature Retinal Cells Retinal Layers Vascular Slabs Schematic Schematic Names and Abbrevations Names and Abbrevations Axon ILM Internal Limiting Membrane OCT Angiography Images Arterioles Vitreous Venules RNFL Cell Body Retinal Nerve Fiber Layer Müller Cell NFLVP GCL Ganglion Cell Layer SVC Dendrite Superficial Synaptic Vascular Terminal SVP Complex IPL Inner Plexiform Layer Axon Capillaries Capillaries Amacrine Cell ICP DVC Cell Body INL Inner Nuclear Layer Deep Inner Retina Dendrite Bipolar Cell Ganglion Cell Vascular NFLVP Capillaries Synaptic Complex Terminal OPL Outer Plexiform Layer DCP Horizontal Cell HFL+ Henle Fiber Layer + Retina Axon ONL Outer Nuclear Layer Müller Cell Cell Body ELM External Limiting Membrane AC Avascular SVP SVC Inner Photoreceptor Rod Complex Cone Segment EZ PR1 Layer of Inner Segment and Outer Retina Ellipsoid Zone Outer Segment Outer IZ PR2 Interdigitation Z. Segment Retinal Pigment Epithelium RPE RPE Bruch‘s Membrane BM Choriocapillaris Choriocapillaris BM CC Capillaries ICP Venules Arterioles Medium and Large Medium and Large CV Choroidal Choroidal Vessels Vessels Choroid Arteries Veins 210250-001 GL.AE20 © Heidelberg Engineering GmbH 210250-001 DCP DVC Fovea Optic Nerve Head www.he-academy.com Axon ILM Arterioles Internal Limiting Membrane Venules RNFL Cell Body Retinal Nerve Fiber Layer Müller Cell NFLVP GCL Ganglion Cell Layer SVC Dendrite Superficial Synaptic Vascular Terminal SVP Complex IPL Inner Plexiform Layer Axon Capillaries Capillaries Amacrine Cell ICP DVC Cell Body INL Inner Nuclear Layer Deep Inner Retina Dendrite Bipolar Cell Ganglion Cell Vascular Capillaries Synaptic Complex Terminal OPL Outer Plexiform Layer DCP Horizontal Cell HFL+ Henle Fiber Layer + Retina Axon ONL Outer Nuclear Layer Müller Cell Cell Body ELM External Limiting Membrane AC Avascular Inner Photoreceptor Rod Segment Complex Cone EZ PR1 Layer of Inner Segment and Outer Retina Ellipsoid Zone Outer Segment Outer IZ PR2 Interdigitation Z. -

Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario Regina (Fowler, 1934)
Kasetsart J. (Nat. Sci.) 49 : 711 - 716 (2015) Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario regina (Fowler, 1934) Piyakorn Boonyoung1, Sinlapachai Senarat2, Jes Kettratad2, Pisit Poolprasert3, Watiporn Yenchum4 and Wannee Jiraungkoorskul5,* ABSTRACT The eye structures and chemical details of the retinal layer in juvenile Queen Danio—Devario regina, an ornamental fish—were histologically investigated under a light microscope. Sample fish were collected from the Tapee River, Nakhon Si Thammarat province, Thailand and their heads were exclusively prepared using a standard histological technique. The results revealed that the eye of D. regina was composed of three layers—inner, middle and external—based on histological organization and cell types. The inner layer was composed of 10 layers; 1) pigment epithelium, 2) photoreceptor layer, 3) outer liming membrane, 4) outer nuclear layer, 5) outer plexiform layer, 6) inner nuclear layer, 7) inner plexiform layer, 8) ganglion cell layer, 9) optic nerve layer and 10) inner limiting membrane, respectively. The localization and chemical details showed that a periodic acid-Schiff reaction for the detection of glycoprotein was intensive in the pigment epithelial layer whereas the inner plexifrom layer had only a slight reaction. Reaction of aniline blue was employed for the detection of mucopolysaccharide which was slightly positive for three layers—the outer limiting membrane, outer plexiform and inner plexiform. Keywords: eye, histology, Devario regina, histochemistry INTRODUCTION cornea and the surrounding water and therefore, the lens has to do the majority of the refraction The eye is a specialized organ for the (Land and Nilsson, 2012). “Due to a refractive detection and analysis of light. -

Retinal Diseases
Over 260 OCT Images Optical Coherence Tomography in Retinal Diseases A Practical Interactive Book For Technicians And Retinal Clinicians Mariano Cozzi Alessandro Invernizzi Carlo Domenico Bianchi Supervised By: E. Priel - G. Staurenghi COPYRIGHT Mariano Cozzi Alessandro Invernizzi Carlo Domenico Bianchi i Main Authors Mariano Cozzi Alessandro Invernizzi Carlo Domenico Bianchi Contributors Alessandro Pagani Sergio Pagliarini Ethan Priel Supervisor Ethan Priel Giovanni Staurenghi Between flesh and what’s fantasy B. Springsteen ii AUTHORS AFFILIATIONS Mariano Cozzi Orthoptist/Research Ophthalmic Photographer, Eye Clinic, Department of Biomedical and Clinical Sciences "Luigi Sacco" Sacco Hospital, Univer- sity of Milan, Milano, Italy. Alessandro Invernizzi MD Ophthalmologist, medical retina and uveitis service, Eye Clinic, Depart- ment of Biomedical and Clinical Sciences "Luigi Sacco" Sacco Hospital, University of Milan, Milano, Italy. Carlo Domenico Bianchi Orthoptist, Eye Clinic, Department of Biomedical and Clinical Sciences "Luigi Sacco" Sacco Hospital, University of Milan, Milano, Italy. Alessandro Pagani Orthoptist, Eye Clinic, Department of Biomedical and Clinical Sciences "Luigi Sacco" Sacco Hospital, University of Milan, Milano, Italy. Sergio Pagliarini MD Ophthalmologist, Retinal Consultant, The Macular Unit, University Hos- pital Coventry and Warwickshire, Coventry, United Kingdom. Ethan Priel Director / Chief Ophthalmic Photographer, Ophthalmology Department, MOR, Bnei Brak, Israel. Giovanni Staurenghi MD Professor of Ophthalmology, -

Nitric Oxide Synthase in Miiller Cells and Neurons of Salamander and Fish Retina
The Journal of Neuroscience, December 1994, 14(12): 7641-7654 Nitric Oxide Synthase in Miiller Cells and Neurons of Salamander and Fish Retina 6. A. Liepe,’ C. Stone,’ J. Koistinaho,’ and D. R. Copenhagen’ ‘Departments of Ophthalmology and Physiology, University of California, San Francisco, California 94143 and 2Department of Biochemistry & Biotechnology, University of Kuopio, FIN-7021 1 Kuopio, Finland Nitric oxide synthase (NOS) is the biosynthetic enzyme of molecule is synthesizedfrom I-arginine by nitric oxide synthase the signaling molecule nitric oxide (NO). NO donors have (NOS; Deguchi and Yoshioka, 1982; Palmer et al., 1988), and been reported to modulate conductances in cell types has been shown to activate soluble guanylyl cyclase (Arnold et throughout the retina, from photoreceptors to ganglion cells. al., 1977) and increaseADP-ribosylation of specificprotein sub- Previously, NOS immunoreactivity has been reported in ama- strates (Brtine and Lapetina, 1989; Zhang et al., 1994) in ap- crine cells and cells within the ganglion cell layer. Here, we propriate target cells. BecauseNO is a short-lived gaseousmol- have examined the cellular localization of NOS in the retinas ecule, it is generally assumedthat the synthetic enzyme NOS of salamander, goldfish, and catfish using both an affinity- must be located either in the target cells or in cells that are purified antiserum to brain NOS and NADPH diaphorase immediately adjacent to the target cells (for review, seeGarth- (NADPHd) histochemistry. These markers indicate that an Waite, 1991). Knowledge of the cell types containing NOS is an NOS-like enzyme is localized not only to presumptive ama- important factor in determining whether NO acts as a neuro- crine cells but also, depending on the species, to photore- modulator in viva The cellular localization of NOS has relied ceptor ellipsoids, to somata within the ganglion cell layer, heavily on both antisera directed against specific isoforms of and to horizontal cells.