Radial and Tangential Dispersion Patterns in the Mouse Retina Are Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Organization of the Inner Nuclear Layer of the Rabbit Retina
The Journal of Neuroscience. January 1995. 15(l): 875-888 The Organization of the Inner Nuclear Layer of the Rabbit Retina Enrica Strettoil and Richard H. Masland2 ‘Istituto di Neurofisiologia del C.N.R., Pisa, Italy; 2Program in Neuroscience and Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts The initial goal of this study was to establish an accounting function (reviewed by Kolb et al., 198 1; Masland, 1988; Cohen of the major classes of cells present in the inner nuclear and Sterling, 1990; WHssle and Boycott, 199 1; Masland, 1992). layer (INL) of the rabbit’s retina. Series of 80-100 radial What one does not know is how many more cell types there sections 1 pm thick were cut from retinal blocks dissected are-how many cells have not yet been encountered by current, at intervals along the vertical meridian. They were photo- essentially trial and error, methods. graphed at high magnification in the light microscope. By The missing cells-those invisible to present staining tech- visualizing the initial segments of processes leaving the so- niques-are important. They represent unseen actors in the ret- mata, we could identify each cell as a bipolar, amacrine, ina’s circuitry. The responses of the retinal ganglion cells are horizontal, or Muller cell. The identifications made by light controlled by all of the neurons afferent to them. If there are microscopy were confirmed by electron microscopy of al- unseen elements afferent to the ganglion cell, our understanding ternating ultrathin sections. On average, bipolar cells made of the creation of ganglion cell responses risks major error. -

Paraneoplastic Retinopathy Associated with Metastatic Cutaneous Melanoma of Unknown Primary Site
PARANEOPLASTIC RETINOPATHY ASSOCIATED WITH METASTATIC CUTANEOUS MELANOMA OF UNKNOWN PRIMARY SITE l 2 l I HA AM KIRATLI , CHARLES E. THIRKILL , SEVGUL BILGI(: , BORA ELDEM YY 1 and ARMAN KE(:ECI Ankara, Turkey and Sacramento, California SUMMARY features of a patient with this rare syndrome are Purpose: To describe further the clinical and immuno described here. logical features of cutaneous melanoma-associated retinopathy, which is an infrequent form of paraneo CASE REPORT plastic syndrome. Methods: We studied the salient clinical and immuno A 66-year-old man without any prior systemic or logical aspects of a 66-year-old man with metastatic ocular problems presented with the complaint of cutaneous melanoma to lymph nodes of unknown mild visual loss of recent onset in his left eye. He had primary site who developed melanoma-associated experienced occasional flashing lights but had no retinopathy. difficulty with night vision. A few days earlier an Results: There was gradual loss of vision in the left eye. incisional biopsy had been done from his right Colour vision and night vision were not affected. Visual axillary region, where rapid enlargement of four or fields showed arcuate defects. A full-field electroretino five lymph nodes each measuring 3 X 2 X 2 cm was gram demonstrated attenuation of the b-wave ampli noticed. tude in the left eye. The a-wave was intact. Indirect His best corrected visual acuity was 6/9 in the right immunofluorescence techniques showed that the anti eye and 6/18 in the left eye. There was no afferent body reactions took place mainly in the outer plexiform pupillary defect. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Microscopic Anatomy of Ocular Globe in Diurnal Desert Rodent Psammomys Obesus (Cretzschmar, 1828) Ouanassa Saadi-Brenkia1,2* , Nadia Hanniche2 and Saida Lounis1,2
Saadi-Brenkia et al. The Journal of Basic and Applied Zoology (2018) 79:43 The Journal of Basic https://doi.org/10.1186/s41936-018-0056-0 and Applied Zoology RESEARCH Open Access Microscopic anatomy of ocular globe in diurnal desert rodent Psammomys obesus (Cretzschmar, 1828) Ouanassa Saadi-Brenkia1,2* , Nadia Hanniche2 and Saida Lounis1,2 Abstract Background: The visual system of desert rodents demonstrates a rather high degree of development and specific features associated with adaptation to arid environment. The aim of this study is to carry out a descriptive investigation into the most relevant features of the sand rat eye. Results: Light microscopic observations revealed that the eye of Psammomys obesus diurnal species, appears similar to that of others rodent with characteristic mammalian organization. The eye was formed by the three distinct layers typical in vertebrates: fibrous tunic (sclera and cornea); vascular tunic (Iris, Ciliary body, Choroid); nervous tunic (retina). Three chambers of fluid fundamentals in maintaining the eyeball’s normal size and shape: Anterior chamber (between cornea and iris), Posterior chamber (between iris, zonule fibers and lens) and the Vitreous chamber (between the lens and the retina) The first two chambers are filled with aqueous humor whereas the vitreous chamber is filled with a more viscous fluid, the vitreous humor. These fluids are made up of 99.9% water. However, the main features, related to life style and arid environment, are the egg-shaped lens, the heavy pigmentation of the middle layer and an extensive folding of ciliary processes, thus developing a large surface area, for ultrafiltration and active fluid transport, this being the actual site of aqueous production. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

Primary Retinal Pathology in Multiple Sclerosis As Detected by Optical Coherence Tomography Downloaded From
doi:10.1093/brain/awq346 Brain 2011: 134; 518–533 | 518 BRAIN A JOURNAL OF NEUROLOGY Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography Downloaded from Shiv Saidha,1 Stephanie B. Syc,1 Mohamed A. Ibrahim,2 Christopher Eckstein,1 Christina V. Warner,1 Sheena K. Farrell,1 Jonathan D. Oakley,3 Mary K. Durbin,3 Scott A. Meyer,3 Laura J. Balcer,4 Elliot M. Frohman,5 Jason M. Rosenzweig,1 Scott D. Newsome,1 John brain.oxfordjournals.org N. Ratchford,1 Quan D. Nguyen2 and Peter A. Calabresi1 1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Carl Zeiss Meditec Inc, Dublin, CA, USA 4 Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA 5 Department of Neurology and Ophthalmology, University of Texas Southwestern, Dallas, TX, USA at University of Texas Southwestern Medical Center Dallas on February 23, 2011 Correspondence to: Dr Peter A. Calabresi, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 627, Baltimore, MD 21287, USA E-mail: [email protected] Optical coherence tomography studies in multiple sclerosis have primarily focused on evaluation of the retinal nerve fibre layer. The aetiology of retinal changes in multiple sclerosis is thought to be secondary to optic nerve demyelination. The objective of this study was to use optical coherence tomography to determine if a subset of patients with multiple sclerosis exhibit primary retinal neuronopathy, in the absence of retrograde degeneration of the retinal nerve fibre layer and to ascertain if such patients may have any distinguishing clinical characteristics. -

Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy
Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy Mathias Abegg, MD, PhD,1 Muriel Dysli,1 Sebastian Wolf, MD, PhD,1 Jens Kowal, PhD,2 Pascal Dufour, MSc,2 Martin Zinkernagel, MD, PhD1 Purpose: To investigate retrograde axonal degeneration for its potential to cause microcystic macular edema (MME), a maculopathy that has been previously described in patients with demyelinating disease. To identify risk factors for MME and to expand the anatomic knowledge on MME. Design: Retrospective case series. Participants: We included 117 consecutive patients and 180 eyes with confirmed optic neuropathy of variable etiology. Patients with glaucoma were excluded. Methods: We determined age, sex, visual acuity, etiology of optic neuropathy, and the temporal and spatial characteristics of MME. Eyes with MME were compared with eyes with optic neuropathy alone and to healthy fellow eyes. With retinal layer segmentation we quantitatively measured the intraretinal anatomy. Main Outcome Measures: Demographic data, distribution of MME in the retina, and thickness of retinal layers were analyzed. Results: We found MME in 16 eyes (8.8%) from 9 patients, none of whom had multiple sclerosis or neu- romyelitis optica. The MME was restricted to the inner nuclear layer (INL) and had a characteristic perifoveal circular distribution. Compared with healthy controls, MME was associated with significant thinning of the ganglion cell layer and nerve fiber layer, as well as a thickening of the INL and the deeper retinal layers. Youth is a significant risk factor for MME. Conclusions: Microcystic macular edema is not specific for demyelinating disease. It is a sign of optic neuropathy irrespective of its etiology. -
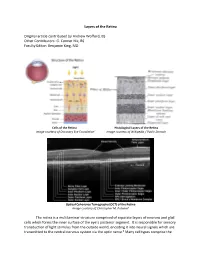
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath. -

Choroidal Morphology and the Outer Choroidoscleral Boundary in Dry Age Related Maculopathy in Swept Source Optical Coherence Tomography
PRACE ORYGINALNE (44) Choroidal morphology and the outer choroidoscleral boundary in dry age related maculopathy in Swept Source Optical Coherence Tomography Ocena morfologii naczyniówki oraz granicy naczyniówkowo-twardówkowej za pomocą optycznej koherentnej tomografii z użyciem lasera strojonego u chorych na suchą postać zwyrodnienia plamki związanego z wiekiem Magdalena Trebinska1, Janusz Michalewski1,2, Jerzy Nawrocki1,2, Zofia Michalewska1,2 1 Dr K. Jonscher Municipal Medical Centre, Lodz, Poland 2 Ophthalmic Clinic “Jasne Blonia”, Lodz, Poland Head: Zofia Michalewska MD, PhD Abstract: Background and objective: To report on details of the choroid in dry age-related macular degeneration imaged with Swept Sour- ce Optical Coherence Tomography. Material and methods: Swept Source Optical Coherence Tomography was performed in 28 eyes with dry age-related macular degeneration (Group 1), who were age and refractive error-matched with 28 eyes of 28 healthy controls (Group 2). The visibility and contour of the outer choroidoscleral boundary and suprachoroidal layer was estimated. Results: Choroidoscleral boundary was visible in all eyes, irregular in 78% in Group 1 and in 18% in Group 2. Suprachoroidal layer was visible in 36% eyes in Group 1 and in 7% eyes in Group 2. Mean choroidal thickness did not differ between groups (p=.11). Conclusions: The outer choroidoscleral boundary is irregular in most cases of age-related macular degeneration. Suprachoroidal layer and suprachoroidal space are more often visible in dry age-related macular degeneration than in healthy controls. Key words: Swept Source Optical Coherence Tomography (SS-OCT), age-related macular degeneration (AMD), choroidal boundary (CSB), suprachoroidal layer (SCL). Abstrakt: Cel: ocena naczyniówki u chorych na suchą postać zwyrodnienia plamki związanego z wiekiem za pomocą optycznej koherent- nej tomografii z użyciem lasera strojonego. -

Download PDF File
Folia Morphol. Vol. 78, No. 2, pp. 237–258 DOI: 10.5603/FM.a2018.0075 O R I G I N A L A R T I C L E Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Aging changes in the retina of male albino rat: a histological, ultrastructural and immunohistochemical study M.E.I. Mohamed, E.A.A. El-Shaarawy, M.F. Youakim, D.M.A. Shuaib, M.M. Ahmed Department of Anatomy and Embryology, Faculty of Medicine, Cairo University, Cairo, Egypt [Received: 9 June 2018; Accepted: 26 June 2018] Background: Degenerative changes caused by aging may affect the eye, especially the retina. Such changes occur as a part of normal physiological process and may be irreversible. The aim of the study was to demonstrate the influence of aging on the morphology of the retina to provide a basis to explain the pathogenesis of age-associated decline in visual acuity, scotopic and photopic sensitivity. Materials and methods: Forty male albino rats were used and divided into four age groups (group I: age of cortical maturity, group II: middle-aged, group III: aged group and group IV: senile group). The rats were sacrificed, the eye balls were enucleated. Intra-vitreal injections of formalin for haematoxylin and eosin and immunohistochemical sections, glutaraldehyde for toluidine blue semithin and E/M ultra-thin sections were performed. Measurements and quantitative histomorphometric estimation of the layers of the retina were done. Results: Light microscopic examination revealed age-dependent attenuation of photoreceptor striations. Aged and senile groups presented pyknotic, widely- -spaced nuclei of the outer nuclear layer. -

Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario Regina (Fowler, 1934)
Kasetsart J. (Nat. Sci.) 49 : 711 - 716 (2015) Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario regina (Fowler, 1934) Piyakorn Boonyoung1, Sinlapachai Senarat2, Jes Kettratad2, Pisit Poolprasert3, Watiporn Yenchum4 and Wannee Jiraungkoorskul5,* ABSTRACT The eye structures and chemical details of the retinal layer in juvenile Queen Danio—Devario regina, an ornamental fish—were histologically investigated under a light microscope. Sample fish were collected from the Tapee River, Nakhon Si Thammarat province, Thailand and their heads were exclusively prepared using a standard histological technique. The results revealed that the eye of D. regina was composed of three layers—inner, middle and external—based on histological organization and cell types. The inner layer was composed of 10 layers; 1) pigment epithelium, 2) photoreceptor layer, 3) outer liming membrane, 4) outer nuclear layer, 5) outer plexiform layer, 6) inner nuclear layer, 7) inner plexiform layer, 8) ganglion cell layer, 9) optic nerve layer and 10) inner limiting membrane, respectively. The localization and chemical details showed that a periodic acid-Schiff reaction for the detection of glycoprotein was intensive in the pigment epithelial layer whereas the inner plexifrom layer had only a slight reaction. Reaction of aniline blue was employed for the detection of mucopolysaccharide which was slightly positive for three layers—the outer limiting membrane, outer plexiform and inner plexiform. Keywords: eye, histology, Devario regina, histochemistry INTRODUCTION cornea and the surrounding water and therefore, the lens has to do the majority of the refraction The eye is a specialized organ for the (Land and Nilsson, 2012). “Due to a refractive detection and analysis of light.