Aging and Degeneration of the Human Macula. 1. Outer Nuclear Layer and Photoreceptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Organization of the Inner Nuclear Layer of the Rabbit Retina
The Journal of Neuroscience. January 1995. 15(l): 875-888 The Organization of the Inner Nuclear Layer of the Rabbit Retina Enrica Strettoil and Richard H. Masland2 ‘Istituto di Neurofisiologia del C.N.R., Pisa, Italy; 2Program in Neuroscience and Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts The initial goal of this study was to establish an accounting function (reviewed by Kolb et al., 198 1; Masland, 1988; Cohen of the major classes of cells present in the inner nuclear and Sterling, 1990; WHssle and Boycott, 199 1; Masland, 1992). layer (INL) of the rabbit’s retina. Series of 80-100 radial What one does not know is how many more cell types there sections 1 pm thick were cut from retinal blocks dissected are-how many cells have not yet been encountered by current, at intervals along the vertical meridian. They were photo- essentially trial and error, methods. graphed at high magnification in the light microscope. By The missing cells-those invisible to present staining tech- visualizing the initial segments of processes leaving the so- niques-are important. They represent unseen actors in the ret- mata, we could identify each cell as a bipolar, amacrine, ina’s circuitry. The responses of the retinal ganglion cells are horizontal, or Muller cell. The identifications made by light controlled by all of the neurons afferent to them. If there are microscopy were confirmed by electron microscopy of al- unseen elements afferent to the ganglion cell, our understanding ternating ultrathin sections. On average, bipolar cells made of the creation of ganglion cell responses risks major error. -

Radial and Tangential Dispersion Patterns in the Mouse Retina Are Cell
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2494-2498, March 1995 Neurobiology Radial and tangential dispersion patterns in the mouse retina are cell-class specific (cell migration/cell lineage/retinal development/transgenic mice/X chromosome inactivation) B. E. REESE*, A. R. HARvEyt, AND S.-S. TANt§ *Neuroscience Research Institute and Department of Psychology, University of California, Santa Barbara, CA 93106; tDepartment of Anatomy and Human Biology, University of Western Australia, Nedlands, WA 6009 Australia; and tDepartment of Anatomy and Cell Biology, University of Melbourne, Parkville, Victoria 3052, Australia Communicated by Pasko Rakic, Yale University School ofMedicine, New Haven, CT, December 16, 1994 ABSTRACT The retina is derived from a pseudostratified retinal cells remain clonally segregated, they should appear as germinal zone in which the relative position of a progenitor distinct groups of blue versus white cells. We have used this cell is believed to determine the position ofthe progeny aligned approach to address the issue of whether radially aligned cells in the radial axis. Such a developmental mechanism would in the mature retina reflect such a clonal derivation. ensure that radial arrays of cells which comprise functional units in the mature central nervous system are also clonally MATERIALS AND METHODS related. The present study has tested this hypothesis by using Retinas from adult transgenic mice, derived from founder line X chromosome-inactivation transgenic mosaic mice. We re- H253, which carries a lacZ transgene -

PARS PLANA VITRECTOMY, RIGHT EYE a Case Study on the Operating
PARS PLANA VITRECTOMY, RIGHT EYE A Case Study on the Operating Room Presented to The Faculty of School of Nursing University of Baguio In Partial fulfillment of the Requirement for the Subject NCENL06 SUBMITTED TO: Larry Michelle Pascual, RN Clinical Instructor SUBMITTED BY: Arlene Esilen Carreon September 2012 1 ACKNOWLEDGMENT I owe my deepest gratitude to the following for the making of this case possible: First and foremost to our Creator, as source of our life and being, and for reasons too numerous to mention; To the University of Baguio, for being true to its mission and vision of empowering its students, giving us the chance to develop our skills through experience; To the Dean, Ms. Jocelyn Apalla, Department Head, Ms. Helen Alalag, and BSN IV Coordinator, Ms. Minda Bahug for making hospital exposure feasible; To my clinical instructor, Mr. Larry Michelle Pascual, who’s intellectual, clinical and practical insights and guidance made our hospital duty experience appreciated and valued in all dimensions; To my parents, for their unending love and support, and for molding me to become the person that I am right now, for the encouragement and words of wisdom they have inculcated in my mind, and the lessons they have taught that help me go on in this part of my journey in life, my deepest gratitude. 2 TABLE OF CONTENTS Chapter Page Title page........................................... i Acknowledgement...................................... ii Table of Content .................................... iii Chapter I Patient’s Profile............................... 1 a. Biographic Data Chapter II Anatomy and Physiology.............................. 2 a. Structure of the Human Eye Chapter III Pathophysiology...................................... 15 Chapter IV Patient’s Preparation............................... -

Paraneoplastic Retinopathy Associated with Metastatic Cutaneous Melanoma of Unknown Primary Site
PARANEOPLASTIC RETINOPATHY ASSOCIATED WITH METASTATIC CUTANEOUS MELANOMA OF UNKNOWN PRIMARY SITE l 2 l I HA AM KIRATLI , CHARLES E. THIRKILL , SEVGUL BILGI(: , BORA ELDEM YY 1 and ARMAN KE(:ECI Ankara, Turkey and Sacramento, California SUMMARY features of a patient with this rare syndrome are Purpose: To describe further the clinical and immuno described here. logical features of cutaneous melanoma-associated retinopathy, which is an infrequent form of paraneo CASE REPORT plastic syndrome. Methods: We studied the salient clinical and immuno A 66-year-old man without any prior systemic or logical aspects of a 66-year-old man with metastatic ocular problems presented with the complaint of cutaneous melanoma to lymph nodes of unknown mild visual loss of recent onset in his left eye. He had primary site who developed melanoma-associated experienced occasional flashing lights but had no retinopathy. difficulty with night vision. A few days earlier an Results: There was gradual loss of vision in the left eye. incisional biopsy had been done from his right Colour vision and night vision were not affected. Visual axillary region, where rapid enlargement of four or fields showed arcuate defects. A full-field electroretino five lymph nodes each measuring 3 X 2 X 2 cm was gram demonstrated attenuation of the b-wave ampli noticed. tude in the left eye. The a-wave was intact. Indirect His best corrected visual acuity was 6/9 in the right immunofluorescence techniques showed that the anti eye and 6/18 in the left eye. There was no afferent body reactions took place mainly in the outer plexiform pupillary defect. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Microscopic Anatomy of Ocular Globe in Diurnal Desert Rodent Psammomys Obesus (Cretzschmar, 1828) Ouanassa Saadi-Brenkia1,2* , Nadia Hanniche2 and Saida Lounis1,2
Saadi-Brenkia et al. The Journal of Basic and Applied Zoology (2018) 79:43 The Journal of Basic https://doi.org/10.1186/s41936-018-0056-0 and Applied Zoology RESEARCH Open Access Microscopic anatomy of ocular globe in diurnal desert rodent Psammomys obesus (Cretzschmar, 1828) Ouanassa Saadi-Brenkia1,2* , Nadia Hanniche2 and Saida Lounis1,2 Abstract Background: The visual system of desert rodents demonstrates a rather high degree of development and specific features associated with adaptation to arid environment. The aim of this study is to carry out a descriptive investigation into the most relevant features of the sand rat eye. Results: Light microscopic observations revealed that the eye of Psammomys obesus diurnal species, appears similar to that of others rodent with characteristic mammalian organization. The eye was formed by the three distinct layers typical in vertebrates: fibrous tunic (sclera and cornea); vascular tunic (Iris, Ciliary body, Choroid); nervous tunic (retina). Three chambers of fluid fundamentals in maintaining the eyeball’s normal size and shape: Anterior chamber (between cornea and iris), Posterior chamber (between iris, zonule fibers and lens) and the Vitreous chamber (between the lens and the retina) The first two chambers are filled with aqueous humor whereas the vitreous chamber is filled with a more viscous fluid, the vitreous humor. These fluids are made up of 99.9% water. However, the main features, related to life style and arid environment, are the egg-shaped lens, the heavy pigmentation of the middle layer and an extensive folding of ciliary processes, thus developing a large surface area, for ultrafiltration and active fluid transport, this being the actual site of aqueous production. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

組織學 Histology 台北醫學大學/解剖學科 教授:邱瑞珍 分機號碼:3261 電子郵件信箱:[email protected]
組織學 Histology 台北醫學大學/解剖學科 教授:邱瑞珍 分機號碼:3261 電子郵件信箱:[email protected] 1 Special sense – eye 台北醫學大學/解剖學科 馮琮涵 副教授 分機3250 E-mail: [email protected] 2 學習目的 •希望同學知道視網膜之顯微構造 •希望同學知道眼瞼顯微結構 3 參考資料 • Junqueira's Basic Histology, twelfth edition, text and atlas, Anthony L. Mescher, McGraw-Hill Companies 4 Summary • A) eye ball: – tunica fibrosa – cornea and sclera – tunica vasculosa—choroids, ciliary process, iris – tunica interna—retina – Lens – vitrous body • B) accessory structure: – eyelids – lacrimal gland 5 6 7 8 Eye • Wall of eyeball: tunicatunica fibrosafibrosa – cornea and sclera tunicatunica vasculosavasculosa – choroid, ciliary body & iris tunicatunica nervosanervosa – retina & pigmented layer opticoptic nervenerve & oraora serrataserrata • Contents: lens, aqueous humor & vitreous body 9 10 Cornea cornea – transparent, avascular, sensory innervation (CN V1) •• cornealcorneal epitheliumepithelium : non-cornified stratified squamous epi. •• BowmanBowman’’ss membranemembrane : basement membrane •• stromastroma : laminated collagen fibers; muco-polysaccharide matrix •• DescemetDescemet’’ss membranemembrane: basement membrane • endothelium 11 Cornea 12 Sclera sclera – thick c.t. with parallel collagen fibers, insertion site of extrinsic eye muscles • Epi-scleral tissue: loose vascular c.t., attaches conjunctiva • stroma: parallel collagen fibers Corneo-scleral junction (limbus): • trabecular meshwork, • canal of Schlemm (scleral venous sinus) 13 14 trabecular meshwork 15 Choroid choroid – soft and thin c.t. membrane • Supra-choroid layer : -

Primary Retinal Pathology in Multiple Sclerosis As Detected by Optical Coherence Tomography Downloaded From
doi:10.1093/brain/awq346 Brain 2011: 134; 518–533 | 518 BRAIN A JOURNAL OF NEUROLOGY Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography Downloaded from Shiv Saidha,1 Stephanie B. Syc,1 Mohamed A. Ibrahim,2 Christopher Eckstein,1 Christina V. Warner,1 Sheena K. Farrell,1 Jonathan D. Oakley,3 Mary K. Durbin,3 Scott A. Meyer,3 Laura J. Balcer,4 Elliot M. Frohman,5 Jason M. Rosenzweig,1 Scott D. Newsome,1 John brain.oxfordjournals.org N. Ratchford,1 Quan D. Nguyen2 and Peter A. Calabresi1 1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Carl Zeiss Meditec Inc, Dublin, CA, USA 4 Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA 5 Department of Neurology and Ophthalmology, University of Texas Southwestern, Dallas, TX, USA at University of Texas Southwestern Medical Center Dallas on February 23, 2011 Correspondence to: Dr Peter A. Calabresi, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Pathology 627, Baltimore, MD 21287, USA E-mail: [email protected] Optical coherence tomography studies in multiple sclerosis have primarily focused on evaluation of the retinal nerve fibre layer. The aetiology of retinal changes in multiple sclerosis is thought to be secondary to optic nerve demyelination. The objective of this study was to use optical coherence tomography to determine if a subset of patients with multiple sclerosis exhibit primary retinal neuronopathy, in the absence of retrograde degeneration of the retinal nerve fibre layer and to ascertain if such patients may have any distinguishing clinical characteristics. -

Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy
Microcystic Macular Edema Retrograde Maculopathy Caused by Optic Neuropathy Mathias Abegg, MD, PhD,1 Muriel Dysli,1 Sebastian Wolf, MD, PhD,1 Jens Kowal, PhD,2 Pascal Dufour, MSc,2 Martin Zinkernagel, MD, PhD1 Purpose: To investigate retrograde axonal degeneration for its potential to cause microcystic macular edema (MME), a maculopathy that has been previously described in patients with demyelinating disease. To identify risk factors for MME and to expand the anatomic knowledge on MME. Design: Retrospective case series. Participants: We included 117 consecutive patients and 180 eyes with confirmed optic neuropathy of variable etiology. Patients with glaucoma were excluded. Methods: We determined age, sex, visual acuity, etiology of optic neuropathy, and the temporal and spatial characteristics of MME. Eyes with MME were compared with eyes with optic neuropathy alone and to healthy fellow eyes. With retinal layer segmentation we quantitatively measured the intraretinal anatomy. Main Outcome Measures: Demographic data, distribution of MME in the retina, and thickness of retinal layers were analyzed. Results: We found MME in 16 eyes (8.8%) from 9 patients, none of whom had multiple sclerosis or neu- romyelitis optica. The MME was restricted to the inner nuclear layer (INL) and had a characteristic perifoveal circular distribution. Compared with healthy controls, MME was associated with significant thinning of the ganglion cell layer and nerve fiber layer, as well as a thickening of the INL and the deeper retinal layers. Youth is a significant risk factor for MME. Conclusions: Microcystic macular edema is not specific for demyelinating disease. It is a sign of optic neuropathy irrespective of its etiology. -

Canine Retina Has a Primate Fovea-Like Bouquet of Cone Photoreceptors Which Is Affected by Inherited Macular Degenerations
Canine Retina Has a Primate Fovea-Like Bouquet of Cone Photoreceptors Which Is Affected by Inherited Macular Degenerations William A. Beltran1*., Artur V. Cideciyan2*., Karina E. Guziewicz1, Simone Iwabe1, Malgorzata Swider2, Erin M. Scott1, Svetlana V. Savina1, Gordon Ruthel3, Frank Stefano4, Lingli Zhang5, Richard Zorger5, Alexander Sumaroka2, Samuel G. Jacobson2, Gustavo D. Aguirre1 1 Department of Clinical Studies, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 2 Department of Ophthalmology, Scheie Eye Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 3 Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 4 Department of Biochemistry, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 5 Department of Neuroscience, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America Abstract Retinal areas of specialization confer vertebrates with the ability to scrutinize corresponding regions of their visual field with greater resolution. A highly specialized area found in haplorhine primates (including humans) is the fovea centralis which is defined by a high density of cone photoreceptors connected individually to interneurons, and retinal ganglion cells (RGCs) that are offset to form a pit lacking retinal capillaries and inner retinal neurons at its center. In dogs, a local increase in RGC density is found in a topographically comparable retinal area defined as the area centralis. While the canine retina is devoid of a foveal pit, no detailed examination of the photoreceptors within the area centralis has been reported. Using both in vivo and ex vivo imaging, we identified a retinal region with a primate fovea-like cone photoreceptor density but without the excavation of the inner retina. -
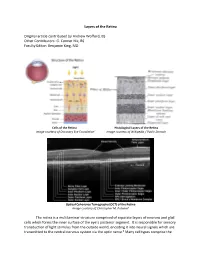
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma.