Australian Trees and Shrubs: Species for Land Rehabilitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ecology Assessment Report Pre-Clearance Survey Report
2 Ecology Assessment Report Pre-clearance Survey Report Consultant/contractor and sub-contractor document review/approval 48DY69, 46DY69, 69DY97, 70DY97, 74DY99, 14DY67, 2RP840942, Warra-Kogan Road reserve, Lot no. Healey's Crossing Road reserve, Dalby-Kogan Road reserve Property name Various Disturbance Tracker no. DP139 Origin/Australia Pacific LNG document no. Q-4331-15-RP-001 Contractor internal reference no. (if 17BRI-7037 applicable) Submitted by (full name of author) Kate Brodie, Loren Appleby, Emma Blacklock Consultant/contractor N/A comments Pre-clearance Survey Report expiry N/A date Technical Revision Date Status Checked Q/A Review 1 12/12/2017 Issued for Use L Appleby L Appleby L Appleby 2 27/08/2018 Issued for Use Liz Fisher Liz Fisher Ailsa Kerswell pp. pp. pp. Template Ref: Q-LNG01-15-AQ-0225 Revision: 2 Approvals, Land and Stakeholder, Australia Pacific LNG Upstream Phase 1 Uncontrolled when printed unless issued and stamped controlled copy. Rev. 0 approved by (name and title) Signature Tim Collins Kainama Development (Stage 1) – Terrestrial Ecology Survey Report Prepared for Origin Energy th 27 August 2018 Kainama Stage 1 Terrestrial Ecology Assessment DOCUMENT TRACKING Item Detail Project Name Kainama Development Stage 1 Ecology Survey Project Number 17BRI-7037 Loren Appleby Project Manager 07 3239 9401 Level 5 / 12 Creek St Brisbane Qld 4000 Prepared by Kate Brodie, Loren Appleby, Emma Blacklock Reviewed by Liz Fisher, Alan House Approved by Ailsa Kerswell Status Final Version Number Revision 2 Last saved on 27th August 2018 Cover Photo Philotheca sporadica and Eucalyputs curtisii, Loren Appleby, 2017. This report should be cited as ‘Eco Logical Australia 2018. -

The Invasion Ecology of Acacia Elata (A
The invasion ecology of Acacia elata (A. Cunn. Ex Benth.) with implications for the management of ornamental wattles by Jason Ernest Donaldson Thesis presented in fulfilment of the requirements for the degree of Masters in the Faculty of Science at Stellenbosch University Supervisor: Prof. David M. Richardson Co-supervisor: Dr. John R. Wilson December 2013 Stellenbosch University http://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety or in part submitted it for obtaining any qualification. September 2013 Copyright © 2013 Stellenbosch University All rights reserved i Stellenbosch University http://scholar.sun.ac.za Abstract This thesis explores how human dictated methods of introduction and species-specific traits interact to define spatial patterns in invasive plant populations using Acacia elata as a model species. I initially asked whether the relatively small invasive extent (when compared to congeners introduced for forestry or dune stabilization) of a species used widely for ornamental purposes (A. elata) is due to low rates of reproduction in South Africa. Results indicate that A. elata has similar traits to other invasive Australia Acacia species: annual seed input into the leaf litter was high (up to 5000 seeds m-2); large seedbanks develop (>20 000 seeds m-2) in established stands; seed germinability is very high (>90%); seeds accumulate mostly in the top soil layers but can infiltrate to depths of 40cm; and seed germination appears to be stimulated by fire. -

Acacia Ammobia Maconochie
WATTLE Acacias of Australia Acacia ammobia Maconochie Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com B.R. Maslin J. & M. Simmons Source: Australian Plant Image Index Image courtesy of Northern Territory Herbarium (dig.32650). ANBG © M. Fagg, 2014 Source: W orldW ideW attle ver. 2. Image courtesy of Northern Territory Herbarium Published at: w w w .w orldw idew attle.com B.R. Maslin Image courtesy of Northern Territory Herbarium Source: Australian Plant Image Index (dig.32646). ANBG © M. Fagg, 2014 Source: Australian Plant Image Index (dig.32648). ANBG © M. Fagg, 2014 Source: Australian Plant Image Index Source: Australian Plant Image Index Source: Australian Plant Image Index (dig.32647). (dig.32649). (dig.32651). ANBG © M. Fagg, 2014 ANBG © M. Fagg, 2014 ANBG © M. Fagg, 2014 Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Image courtesy of Northern Territory Herbarium Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com B.R. Maslin J. & M. Simmons Acacia ammobia occurrence map. O ccurrence map generated via Atlas of Living Australia (https://w w w .ala.org.au). Common Name Mt Conner Wattle Family Fabaceae Distribution Occurs in south-western N.T., from 130 km W to 50 km E of Uluru, and in north-western S.A. -

Native Plants Sixth Edition Sixth Edition AUSTRALIAN Native Plants Cultivation, Use in Landscaping and Propagation
AUSTRALIAN NATIVE PLANTS SIXTH EDITION SIXTH EDITION AUSTRALIAN NATIVE PLANTS Cultivation, Use in Landscaping and Propagation John W. Wrigley Murray Fagg Sixth Edition published in Australia in 2013 by ACKNOWLEDGEMENTS Reed New Holland an imprint of New Holland Publishers (Australia) Pty Ltd Sydney • Auckland • London • Cape Town Many people have helped us since 1977 when we began writing the first edition of Garfield House 86–88 Edgware Road London W2 2EA United Kingdom Australian Native Plants. Some of these folk have regrettably passed on, others have moved 1/66 Gibbes Street Chatswood NSW 2067 Australia to different areas. We endeavour here to acknowledge their assistance, without which the 218 Lake Road Northcote Auckland New Zealand Wembley Square First Floor Solan Road Gardens Cape Town 8001 South Africa various editions of this book would not have been as useful to so many gardeners and lovers of Australian plants. www.newhollandpublishers.com To the following people, our sincere thanks: Steve Adams, Ralph Bailey, Natalie Barnett, www.newholland.com.au Tony Bean, Lloyd Bird, John Birks, Mr and Mrs Blacklock, Don Blaxell, Jim Bourner, John Copyright © 2013 in text: John Wrigley Briggs, Colin Broadfoot, Dot Brown, the late George Brown, Ray Brown, Leslie Conway, Copyright © 2013 in map: Ian Faulkner Copyright © 2013 in photographs and illustrations: Murray Fagg Russell and Sharon Costin, Kirsten Cowley, Lyn Craven (Petraeomyrtus punicea photograph) Copyright © 2013 New Holland Publishers (Australia) Pty Ltd Richard Cummings, Bert -

Acacia Burrowii Maiden
WATTLE Acacias of Australia Acacia burrowii Maiden Source: Australian Plant Image Index Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. Source: Australian Plant Image Index (dig.23654). Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com (dig.23655). ANBG © M. Fagg, 2012 J. & M. Simmons J. & M. Simmons ANBG © M. Fagg, 2012 Source: Australian Plant Image Index Source: W orldW ideW attle ver. 2. Source: W orldW ideW attle ver. 2. (dig.23656). Published at: w w w .w orldw idew attle.com Published at: w w w .w orldw idew attle.com ANBG © M. Fagg, 2012 Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com J. & M. Simmons Source: W orldW ideW attle ver. 2. Published at: w w w .w orldw idew attle.com Acacia burrow ii occurrence map. O ccurrence map generated via Atlas of Living Australia (https://w w w .ala.org.au). Common Name Burrow’s Wattle Family Fabaceae Distribution Occurs on the North Western Plains of N.S.W. from Cobar-Nyngan area N to Yetman (including the Pilliga Scrub), and in south-eastern Qld from the Goodiwindi– Moonie area N to Eidsvold, W of 151ºE. Description Tree to 13 m high, single-stemmed. Bark ribbony, grey. Branchlets angular towards apices, red-brown, scurfy, glabrous, ±resinous. Phyllodes narrowly to very narrowly elliptic, flat, straight or slightly curved, (2–) 3–11 cm long, (2–) 4–10 mm wide (juveniles to 14 mm wide and scurfy), coriaceous, with 1–3 slightly prominent main nerves; minor nerves 8–10 per mm, parallel, not anastomosing; gland 1, basal, to 1 mm above pulvinus. -

Appendices, Glossary and Index
7 Appendices Appendix A: Agency resourcing statement 2010–11 Appendix B: Portfolio Budget Statements reporting 2010–11 Appendix C: Ecologically sustainable development and environmental performance Appendix D: Freedom of information statement Appendix E: Compliance index Christmas Island red crabs. Photo: Parks Australia Appendix A: Agency Resourcing Statement 2010–11 The Agency Resourcing Statement was introduced to Portfolio Budget Statements in 2008–09 to provide information about the various funding sources that the Director of National Parks may draw upon during the year. The Director of National Parks is required to publish the Agency Resourcing Statement in the annual report that reconciles to cash reserves in the financial statements. Actual available Payments Balance appropriation Made Remaining $’000 $’000 $’000 Opening balance/Reserves at bank 38,353 – 38,353 REVENUE FROM GOVERNMENT Ordinary annual services¹ Outcome 1 – – – Total ordinary annual services – – – Other services² Non-operating 2,249 – 2,249 Total other services 2,249 – 2,249 Total annual appropriations 2,249 – 2,249 Payments from related entities3 Amounts from the portfolio department 46,444 46,444 (0) Total 46,444 46,444 (0) Total funds from Government 46,444 46,444 (0) FUNDS FROM OTHER SOURCES Interest 1,575 1,575 – Sale of goods and services 15,486 15,486 – Other 2,328 1,390 938 Total 19,389 18,451 938 Total net resourcing for DNP 106,435 64,895 41,540 All figures are GST exclusive As per the Environment Protection and Biodiversity Conservation Act 1999 Section 514S, DSEWPaC is directly appropriated the Director of National Parks (DNP) appropriations, which is then allocated to the DNP by the Secretary. -
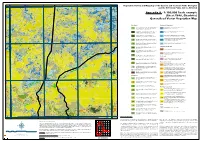
Appendix 9 - 1:100,000 Scale Example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map
133°30'E 133°40'E 133°50'E 134°E 24°30'S Vegetation Survey and Mapping of the Eastern and Southern Finke Bioregion 24°30'S and the NT Stony Plains Inliers, NT & SA Appendix 9 - 1:100,000 Scale example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map Woodland Chenopod Shrubland Acacia aneura ( Mulga) Low Open Woodland TO Tall Open Shrubland of Atriplex nummularia (Old man saltbush) Low Sparse Chenopod 1 Acacia estrophiolata (Ironwood) on clay loam plains and red earth 4 shrubland over Low Sparse Tussock grasses. soils+/- Atriplex vesicaria and Eragrostis eriopoda . Acacia georginae / Acacia cambagei ( Gidgee) Low Woodland to Tall Atriplex vesicaria (Pop saltbush) Low Open Chenopod Shrubland.+/- 2 Shrubland.+/- Eucalyptus coolabah subsp. Arida , Codonocarpus 5 Maireana astrotricha over tussock grasses. cotinifolius , Eulalia aurea, Eriachne ovata and Atriplex vesicaria . Eucalyptus coolabah subsp. arida (Coolabah) Woodland. +/- Maireana aphylla (Cottonbush) Low Sparse Chenopod Shrubland. +/- 12 Muehlenbeckia florulenta , Acacia aneura , Senna artemisioides ssp. 8 Fimbristylis dichotoma , Dactyloctenium radulans and Eragrostis dielsii. Filifolia , Marsilea sp ., Cynodon dactylon , and Cenchrus ciliaris . Maireana astrotricha (Low bluebush) Low Sparse Chenopod Shrubland Eucalyptus camaldulensis var. obtusa (River red gum) Woodland.+/- TO Sparse shrubland of Senna artemisioides n. coriacea and 13 Eucalyptus coolabah subsp. arida , Cynodon dactylon , Eulalia aurea and 9 Eremophila duttonii (Harlequin fuchsia bush). Cyperus gymnocaulos . 24°40'S Hakea leucoptera subsp. leucoptera (Needlewood) Open Woodland. +- Sclerolaena (mixed) Low Sparse Chenopod Shrubland.+/- Enneapogon 24°40'S 14 Eremophila sturtii , Senna artemisioides ssp. filifolia , Hakea leucoptera 15 avenaceus Aristida contorta , Sporobolus actinocladus . subsp. leucoptera and Triodia basedowii . Acacia calcicola (Northern Myall) Sparse Woodland +/- Eremophila Samphire Shrubland 23 duttonii , Acacia calcicola , Atriplex vesicaria , Maireana georgei and mixed short grasses. -

Volume 5 Pt 3
Conservation Science W. Aust. 7 (1) : 153–178 (2008) Flora and Vegetation of the banded iron formations of the Yilgarn Craton: the Weld Range ADRIENNE S MARKEY AND STEVEN J DILLON Science Division, Department of Environment and Conservation, Wildlife Research Centre, PO Box 51, Wanneroo WA 6946. Email: [email protected] ABSTRACT A survey of the flora and floristic communities of the Weld Range, in the Murchison region of Western Australia, was undertaken using classification and ordination analysis of quadrat data. A total of 239 taxa (species, subspecies and varieties) and five hybrids of vascular plants were collected and identified from within the survey area. Of these, 229 taxa were native and 10 species were introduced. Eight priority species were located in this survey, six of these being new records for the Weld Range. Although no species endemic to the Weld Range were located in this survey, new populations of three priority listed taxa were identified which represent significant range extensions for these taxa of conservation significance. Eight floristic community types (six types, two of these subdivided into two subtypes each) were identified and described for the Weld Range, with the primary division in the classification separating a dolerite-associated floristic community from those on banded iron formation. Floristic communities occurring on BIF were found to be associated with topographic relief, underlying geology and soil chemistry. There did not appear to be any restricted communities within the landform, but some communities may be geographically restricted to the Weld Range. Because these communities on the Weld Range are so closely associated with topography and substrate, they are vulnerable to impact from mineral exploration and open cast mining. -

Clearing Permit Decision Report
Clearing Permit Decision Report 1. Application details 1.1. Permit application details Permit application No.: 3009/2 Permit type: Purpose Permit 1.2. Proponent details Proponent’s name: Hamersley Iron Pty Ltd 1.3. Property details Property: Iron Ore (Rhodes Ridge ) Agreement Authorisation Act 1972 , Temporary Reserves 70/4192, 70/4266, 70/4267, 70/4737 Local Government Area: Shire of East Pilbara Colloquial name: Geotechnical Test Pitting Project 1.4. Application Clearing Area (ha) No. Trees Method of Clearing For the purpose of: 112 Mechanical Removal Geotechnical Test Pitting 2. Site Information 2.1. Existing environment and information 2.1.1. Description of the native vegetation under application Vegetation Description Beard Vegetation Associations have been mapped at a scale of 1:250,000 for the whole of Western Australia. Three Beard Vegetation Associations are located within the application areas (Shepherd et al., 2001): • Beard Vegetation Association 18: Low woodland; mulga ( Acacia aneura ); • Beard Vegetation Association 29: Sparse low woodland; mulga, discontinuous in scattered groups; and • Beard Vegetation Association 82: Hummock grasslands, low tree steppe; snappy gum over Triodia wiseana . Mattiske Consulting (2008) has conducted a flora and vegetation survey over an area that included the application areas. The survey was conducted in April and May 2008 following favourable seasonal rainfall (Mattiske Consulting, 2008). Mattiske Consulting (2008) has recorded 25 vegetation units within the vegetation survey area with the following 11 being likely to be impacted by the proposed clearing: Flowlines (Creeklines and Drainage Areas): C2) Low woodland of Eucalyptus xerothermica, Eucalyptus victrix over Acacia citrinoviridis and Acacia maitlandii, Gossypium australe, Melaleuca lasiandra, Petalostylis labicheoides, Rulingia luteiflora over Triodia epactia, Chrysopogon fallax and Triodia pungens on minor creeklines with sandy soils. -

Southern Gulf, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

BIODIVERSITY CONSERVATION on the TIWI ISLANDS, NORTHERN TERRITORY: Part 1. Environments and Plants
BIODIVERSITY CONSERVATION ON THE TIWI ISLANDS, NORTHERN TERRITORY: Part 1. Environments and plants Report prepared by John Woinarski, Kym Brennan, Ian Cowie, Raelee Kerrigan and Craig Hempel. Darwin, August 2003 Cover photo: Tall forests dominated by Darwin stringybark Eucalyptus tetrodonta, Darwin woollybutt E. miniata and Melville Island Bloodwood Corymbia nesophila are the principal landscape element across the Tiwi islands (photo: Craig Hempel). i SUMMARY The Tiwi Islands comprise two of Australia’s largest offshore islands - Bathurst (with an area of 1693 km 2) and Melville (5788 km 2) Islands. These are Aboriginal lands lying about 20 km to the north of Darwin, Northern Territory. The islands are of generally low relief with relatively simple geological patterning. They have the highest rainfall in the Northern Territory (to about 2000 mm annual average rainfall in the far north-west of Melville and north of Bathurst). The human population of about 2000 people lives mainly in the three towns of Nguiu, Milakapati and Pirlangimpi. Tall forests dominated by Eucalyptus miniata, E. tetrodonta, and Corymbia nesophila cover about 75% of the island area. These include the best developed eucalypt forests in the Northern Territory. The Tiwi Islands also include nearly 1300 rainforest patches, with floristic composition in many of these patches distinct from that of the Northern Territory mainland. Although the total extent of rainforest on the Tiwi Islands is small (around 160 km 2 ), at an NT level this makes up an unusually high proportion of the landscape and comprises between 6 and 15% of the total NT rainforest extent. The Tiwi Islands also include nearly 200 km 2 of “treeless plains”, a vegetation type largely restricted to these islands. -

MOUNT AMOS South Pacific Ocean
Refer to this map as: Wet Tropics Bioregion MOUNT AMOS 1:50 000 SERIES WTMAveg Vegetation Survey QUEENSLAND SHEET 7966-1 EDITION 1 3 13 14 15 16 17 18 19 20 21145°20' 22 23 24 25 26 27 28 29 330 145°25' 31 32 33 34 35 36 37 38 39 145°30' MOUNT COOK NATIONAL PARK 15°30' 15°30' 43 85 Dawson Reef 85 62 84 56 84 South 83 An nan R 83 54a ive 70e r 42a 70a 70e 54a 54a Osterland Reef 39a 54a 55a 54a 55a 64a 82 54a 54a Cowlishaw Reef 54a 55a 64a 39a 82 70a 70a 70e 54a 70a 70a 70e Esk 70e 70a 70a 59a 81 29b 3a 70e 26a 70a 39a 81 54a 135 68d 70e Mount McIntosh 26a 70a 70e Draper Patch 29b 29b 64a 55a 70e 70e 39a 29b 26a 82 54a 39a 68d 80 21a Grave Point 70e 70e 33a 54a 59a 39a 25c 54a 8280 ANNAN RIVER 68d 54a 68d59b 55a 21a 33a 64a Walker Bay 43 70e 54a R 29b 39a 26a 54a i 47e 59a v 59a 68d e 48a 39a 77 Pacific 70e r 39a 29b 26a 50a 79 59a 70a 70e 26a 68d 29b 26a 70a 26a 70e 54a 59a 79 42a 33a 26a 55a 55a 68d 42a 48a 59a 54a54a 54a 110 48a 33a 55a 68d68d 42a 47e 29b 39a 54a 68d 59a 70e 55a 39a 62a 39a 42a 191 68d 39a 29b 67c 3a 70a 62a 67c Walker Hill 68d 33a 55a 33a 33a 39a 33a 67c 59b 59b 26a 78 NATIONAL PARK 39a 50a 42a 33a 33a 62a 59a 33a 3a 33a 50a 59a 39a Walker Point 78 62a 42a 59a 48a 29b 39a 26a 68d 48a 48a 3a 33a 59a 39a 3a 57a 33a 59b 39a 33a 67c 3a 59a 67c 68d 67c 67c 59a42a 26a 59a 39a 33a 33a 67c 64a 67c 59a 33a 59b 50a 76 57a 48a 67c 26a 77 33a 62a 42a 38 67c 67c 33a 39a 48a 67c 33a 67c 50a 39a 68d 62a 39a 67c 57a 77 61c 33a 29b 48a 67c 57 33a 33a 67c 29b 68d 42a 67c 67c 67c 42a 33a 39a 67c 67c 15°35' 59a 42a 64a 15°35' 50a 48a 33a