An Investigation of the Bragg-Gray Principle with Fluorescent X-Rays
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACUTE RADIATION SYNDROME: Diagnosis and Treatment
ACUTE RADIATION SYNDROME: Diagnosis and Treatment Badria Al Hatali, MD Medical Toxicologist Department of Environmental and Occupational Health MOH - Oman Objectives Provide a review of radiation basics and acute radiation sickness Discuss diagnostic tools and triage tools for Acute Radiation Syndromes Discuss management of Acute Radiation Syndromes Energy traveling over a distance as Waves Particles • Gamma rays • Alpha • X-rays • Beta • Radio waves • neurons Non-ionizing vs Ionizing Radiation • High energy • Low energy • Removes orbital electrons • Does not remove orbital from atoms > DNA electrons from atom damage Radioactive Decay Process to Remove excess energy from atomic nuclei Nuclei emit rays or particles to decrease nuclear energy Radioactive materials have unstable nuclei with excess energy Ionizing Radiation Dose • Radiation absorb dose (RAD): the amount of energy absorbed by the body. 1 cGy = 0.01 J/kg (USA) • Gray (Gy): expressed as absorbed energy per unit mass of tissue. 100 rad =100 cGy =1 J/kg (SI) • Roentgen Equivalent Man (REM) relates the absorbed dose in human tissue to the effective biological damage of the radiation (USA) • Sievert (Sv): the absorbed dose in human tissue to the effective biological damage of the radiation (SI) Radioactivity Biological And Effective Half-lives Biological half-life is the time to remove half of radioactive element from body Effective half-life is the combined effect of radioactive decay & biological elimination Effective half-life is always shorter than either physical or biological half-lives Biological Effects of Ionizing Radiation Direct damage Chromosome Other biochemical E.g. alpha and beta particles Indirect damage Chemical changes due to radiolysis of water in cell E.g. -

Emergency and Combat First Aid» Module № 1 Emergency and Combat First Aid Topic 7 Means of Mass Destruction
Ministry of Health of Ukraine Ukrainian Medical stomatological Academy It is ratified On meeting department Of accident aid and military medicine «___»_____________20 __y. Protocol №_____ Manager of department DMSc ., assistant professor __________К.Shepitko METHODICAL INSTRUCTION FOR INDEPENDENT WORK OF STUDENTS DURING PREPARATIONS FOR THE PRACTICAL LESSON Educational discipline «Emergency and Combat First Aid» Module № 1 Emergency and Combat First Aid Topic 7 Means of Mass Destruction. First Aid. Weapons of mass destructions. Lesson 10 Radiations chemical accidents .First Aid Сourse ІІ Foreing students training dentistry Faculty Training of specialists of the second (master) level of higher of education (название уровня высшего образования) Areas of knowledge _______ 22 «Health protection»_________ (шифр и название области знаний) Specialty ________222 «Medicine», 221 «Stomatology»________________ (код и наименование специальности) Poltava 2019 The relevance of the topic: Military action in modern warfare will be carried out with high activity and limit tension. They cause great losses in the army and among the population, the destruction of potentially dangerous objects, energy centers, waterworks, the formation of large zones of destruction, fires and floods. The main form of countering in the war, is armed struggle - the organized use of armed forces and weapons to achieve specific political and military objectives, a combination of military actions of varying scales. To conventional weapons, the application of which may cause losses among the population are missiles and aerial munitions, including precision munitions volumetric detonation of cluster and incendiary. Have the greatest efficiency high precision conventional weapons, which provide automatic detection and reliable destruction of targets and enemy targets with a single shot (trigger). -
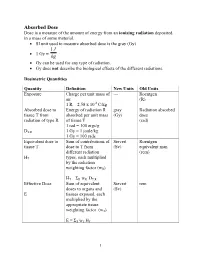
Absorbed Dose Dose Is a Measure of the Amount of Energy from an Ionizing Radiation Deposited in a Mass of Some Material
Absorbed Dose Dose is a measure of the amount of energy from an ionizing radiation deposited in a mass of some material. • SI unit used to measure absorbed dose is the gray (Gy). 1J • 1 Gy = kg • Gy can be used for any type of radiation. • Gy does not describe the biological effects of the different radiations. Dosimetric Quantities Quantity Definition New Units Old Units Exposure Charge per unit mass of --- Roentgen air (R) 1 R = 2.58 x 10-4 C/kg Absorbed dose to Energy of radiation R gray Radiation absorbed tissue T from absorbed per unit mass (Gy) dose radiation of type R of tissue T (rad) 1 rad = 100 ergs/g DT,R 1 Gy = 1 joule/kg 1 Gy = 100 rads Equivalent dose to Sum of contributions of Sievert Roentgen tissue T dose to T from (Sv) equivalent man different radiation (rem) HT types, each multiplied by the radiation weighting factor (wR) HT = ΣR wR DT,R Effective Dose Sum of equivalent Sievert rem doses to organs and (Sv) E tissues exposed, each multiplied by the appropriate tissue weighting factor (wT) E = ΣT wT HT 1 Radiological Protection For practical purposes of assessing and regulating the hazards of ionizing radiation to workers and the general population, weighting factors (previously called quality factors, Q) are used. A radiation weighting factor is an estimate of the effectiveness per unit dose of the given radiation relative a to low-LET standard. Weighting factors are dimensionless multiplicative factors used to convert physical dose (Gy) to equivalent dose (Sv) ; i.e., to place biological effects from exposure to different types of radiation on a common scale. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Office of Radiation Protection
How is Dose Measured? July 2002 Fact Sheet 320-058 Division of Environmental Health Office of Radiation Protection RADIATION DOSE When radioactive material decays and the transformation of the atom occurs there is characteristic energy that is released. This energy is released in the form of what we call radiation. There are different types of radiation, but they all serve the same general purpose, ridding the atom of excess energy after it transforms. These radiations travel until, by losing energy, they “stop”. Radiation loses its energy by interacting with atoms in its pathway and transferring energy to the atom during these interactions. When an interaction with radiation removes an electron from the atom it is called ionization. Other types of interactions include the excitation of an atom, the breaking of molecular bonds, and the heating of an atom or molecule. Ionization, excitation, and molecular bond breaking can cause biological damage; heat transfer does not necessarily cause biological damage. The purely physical event of energy deposited by a radiation in a given volume of material, i.e. tissue, is called the absorbed dose. The unit of absorbed dose is called the Rad, the international unit is the Gray (gy). The absorbed dose quantifies the amount of energy transferred to a volume of material, but it does not reflect the biological damage that potentially occurred. Because of the physics of radiation, the biological effect of the same amount of absorbed energy may vary according to the type of the radiation. A quality factor, Q was developed, to be able to compare absorbed doses from different radiation types. -

Chapter 2: Dosimetricprinciples, Quantitiesandunits
Chapter 2: Dosimetric Principles, Quantities and Units Set of 131 slides based on the chapter authored by J.P. Seuntjens, W. Strydom, and K.R. Shortt of the IAEA publication (ISBN 92-0-107304-6): Review of Radiation Oncology Physics: A Handbook for Teachers and Students Objective: To familiarize the student with the basic principles of the quantities used in dosimetry for ionizing radiation. Slide set prepared in 2006 by G.H. Hartmann (Heidelberg, DKFZ) Comments to S. Vatnitsky: [email protected] Version 2012 IAEA International Atomic Energy Agency CHAPTER 2. TABLE OF CONTENTS 2.1 Introduction 2.2 Radiation field quantities (also denoted as Radiometric quantities) 2.3 Dosimetrical quantities: fundamentals 2.4 Dosimetrical quantities 2.5 Interaction coefficients: electrons 2.6 Interaction coefficients: photons 2.7 Relation between radiation field and dosimetric quantities 2.8 Cavity theory IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 2.Slide 1 2.1 INTRODUCTION Radiation dosimetry has its origin in the medical application of ionizing radiation starting with the discovery of x-rays by Röntgen in 1895. In particular • the need of protection against ionizing radiation, • the application in medicine required quantitative methods to determine a "dose of radiation". The purpose of a quantitative concept of a dose of radiation is: • to predict associated radiation effects (radiation detriments) • to reproduce clinical outcomes. IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 2.1. Slide 1 2.1 INTRODUCTION The connection to the medical profession is obvious. The term dose of radiation was initially used in a pharmacological sense, that means: analogously to its meaning when used in prescribing a dose of medicine. -

Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements?
www.JICReadiness.com Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements? A: Since nuclear radiation affects people, we must be able to measure its presence. We also need to relate the amount of radiation received by the body to its physiological effects. Two terms used to relate the amount of radiation received by the body are exposure and dose. When you are exposed to radiation, your body absorbs a dose of radiation. As in most measurement quantities, certain units are used to properly express the measurement. For radiation measurements they are... Roentgen: The roentgen measures the energy produced by gamma radiation in a cubic centimeter of air. It is usually abbreviated with the capital letter "R". A milliroentgen, or "mR", is equal to one one-thousandth of a roentgen. An exposure of 50 roentgens would be written "50 R". Rad: Or, Radiation Absorbed Dose recognizes that different materials that receive the same exposure may not absorb the same amount of energy. A rad measures the amount of radiation energy transferred to some mass of material, typically humans. One roentgen of gamma radiation exposure results in about one rad of absorbed dose. Rem: Or, Roentgen Equivalent Man is a unit that relates the dose of any radiation to the biological effect of that dose. To relate the absorbed dose of specific types of radiation to their biological effect, a "quality factor" must be multiplied by the dose in rad, which then shows the dose in rems. For gamma rays and beta particles, 1 rad of exposure results in 1 rem of dose. -

Radiation Dose Associated with Common Computed Tomography Examinations and the Associated Lifetime Attributable Risk of Cancer
WEB-ONLY CONTENT Radiation Dose Associated With Common Computed Tomography Examinations and the Associated Lifetime Attributable Risk of Cancer Rebecca Smith-Bindman, MD; Jafi Lipson, MD; Ralph Marcus, BA; Kwang-Pyo Kim, PhD; Mahadevappa Mahesh, MS, PhD; Robert Gould, ScD; Amy Berrington de Gonza´lez, DPhil; Diana L. Miglioretti, PhD Arch Intern Med. 2009;169(22):2078-2086 DOSIMETRY QUANTITIES defined as joules per kilogram. The unit of gray can be used AND GLOSSARY OF TERMS for any type of radiation, but it does not describe the bio- logical effects of different types of radiation. When radiation interacts with biological matter, the re- Radiation Weighting Factor: A dimensionless factor sulting biological effect depends on the amount of ra- by which the organ absorbed dose (rad or gray) is mul- diation energy absorbed into the material and the type tiplied to obtain a quantity that expresses, on a common of radiation. Organ dose or organ-specific absorbed dose scale for all ionizing radiation, the biological damage (rem is defined as the energy imparted per unit mass of an or- or sievert) to an exposed person. It is used because some gan or tissue. The “absorbed dose” is measured in grays types of radiation, such as alpha particles, are more bio- (Gy), defined as joules per kilogram. The gray replaced logically damaging internally than others. It is used to the rad (radiation absorbed dose), the traditional unit of derive the equivalent dose from the absorbed dose aver- absorbed dose, which is equal to 0.01 Gy. aged over a tissue or organ. -

Risk of Acute Radiation Syndromes Due to Solar Particle Events
Evidence Report: Risk of Acute Radiation Syndromes due to Solar Particle Events Human Research Program Space Radiation Program Element Approved for Public Release: April 6, 2016 National Aeronautics and Space Administration Lyndon B. Johnson Space Center Houston, Texas CURRENT CONTRIBUTING AUTHORS: Lisa Carnell, PhD NASA Langley Research Center, Hampton, VA Steve Blattnig, PhD NASA Langley Research Center, Hampton, VA Shaowen Hu, PhD Wyle Science Technology & Engineering, Houston, TX Janice Huff, PhD Universities Space Research Association, Houston, TX Myung-Hee Kim, PhD Wyle Science Technology & Engineering, Houston, TX Ryan Norman, PhD NASA Langley Research Center, Hampton, VA Zarana Patel, PhD Wyle Science Technology & Engineering, Houston, TX Lisa Simonsen, PhD NASA Langley Research Center, Hampton, VA Honglu Wu, PhD NASA Johnson Space Center, Houston, TX PREVIOUS CONTRIBUTING AUTHORS: Honglu Wu NASA Johnson Space Center Janice L. Huff Universities Space Research Association Rachel Casey Universities Space Research Association Myung-Hee Kim Universities Space Research Association Francis A. Cucinotta NASA Johnson Space Center Reference for original report: Human Health and Performance Risks of Space Exploration Missions, (Jancy C. McPhee and John B. Charles, editors), NASA SP-2009- 3405, 2009. 1 Table of Contents I. PRD RISK TITLE: RISK OF ACUTE RADIATION SYNDROMES DUE TO SOLAR PARTICLE EVENTS ........................................................................................... 4 II. EXECUTIVE SUMMARY ................................................................................................ -

Cavity Theory with Corrections
CitThCavity Theory, Stopping -Power Ratios, Correction Factors. Alan E. Nahum PhD Physics Department Clatterbridge Centre for Oncology Bebington, Wirral CH63 4JY UK ((@[email protected]) AAPM Summer School, CLINICAL DOSIMETRY FOR RADIOTHERAPY, 21-25 June 2009, Colorado College, Colorado Springs, USA 1 A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. 313.1 INTRODUCTION 3.2 “LARGE” PHOTON DETECTORS 3.3 BRAGG-GRAY CAVITY THEORY 3.4 STOPPING-POWER RATIOS 3.5 THICK-WALLED ION CHAMBERS 363.6 CORRECTION OR PERTURBATION FACTORS FOR ION CHAMBERS 373.7 GENERAL CAVITY THEORY 3.8 PRACTICAL DETECTORS 3.9 SUMMARY 2 A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. Accurate knowledgg(p)e of the (patient) dose in radiation therapy is crucial to clinical outcome For a given fraction size 100 TCP 80 (%) 60 Therapeutic Ratio NTCP 40 & NTCP 20 TCP 0 20 40 60Dpr 80 100 Dose (Gy) 3 A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. Detectors almost never measure dose to medium directly. Therefore, the interpretation of detector reading requires dosimetry theory - “cavity theory” 4 A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. D especially when converting med from calibration at Q to f Q 1 D measurement at Q2 5 det Q A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. Also the “physics” of depth-dose curves: 6 A. E. Nahum: Cavity Theory, Stopping-Power Ratios, Correction Factors. Dose computation in a TPS Terma cfKf. Kerma 7 First we will remind ourselves of two key results which relate the particle fluence, , to energy deposition in the medium. -

Acute Radiation Syndrome: a Fact Sheet for Physicians
FACT SHEET Acute Radiation Syndrome: A Fact Sheet for Physicians Acute Radiation Syndrome (ARS) (sometimes known as radiation toxicity or radiation sickness) is an acute illness caused by irradiation of the entire body (or most of the body) by a high dose of penetrating radiation in a very short period of time (usually a matter of minutes). The major cause of this syndrome is depletion of immature parenchymal stem cells in specific tissues. Examples of people who suffered from ARS are the survivors of the Hiroshima and Nagasaki atomic bombs, the firefighters that first responded after the Chernobyl Nuclear Power Plant event in 1986, and some unintentional exposures to sterilization irradiators. The required conditions for Acute Radiation Syndrome (ARS) are: • The radiation dose must be large (i.e., greater than 0.7 Gray (Gy)1,2 or 70 rads). o Mild symptoms may be observed with doses as low as 0.3 Gy or 30 rads. • The dose usually must be external (i.e., the source of radiation is outside of the patient’s body). o Radioactive materials deposited inside the body have produced some ARS effects only in extremely rare cases. • The radiation must be penetrating (i.e., able to reach the internal organs). o High energy X-rays, gamma rays, and neutrons are penetrating radiations. • The entire body (or a significant portion of it) must have received the dose.3 o Most radiation injuries are local, frequently involving the hands, and these local injuries seldom cause classical signs of ARS. • The dose must have been delivered in a short time (usually a matter of minutes). -

10. Cavity Theory
Outline • Problem statement Cavity Theory • Bragg-Gray cavity theory • Spencer cavity theory Chapter 10 • Burlin cavity theory • Dose near interfaces between F.A. Attix, Introduction to Radiological dissimilar media Physics and Radiation Dosimetry • Summary Cavity Theory: Problem Statement Bragg-Gray Theory • Homogeneous medium, Q wall (w) • Probe - cavity - thin layer of gas (g) • Charged particles crossing + - w-g interface gas • Objective: find a relation Dg between the dose in a probe wall to that in the medium (A) A fluence of charged particles crossing an interface between media w and g Dw (B) A fluence of charged particles passing through a thin layer of medium g • Basis for dosimetry sandwiched between regions contain medium w Fluence is assumed to be continuous across all interfaces; it is related to the dose Bragg-Gray Theory Bragg-Gray Theory • Charged particles of fluence • Two conditions: , kinetic energy T – Thickness of g layer is much smaller than the range of charged particles (medium g is close to w in atomic • Dg, Dw - absorbed doses on number) each side of the boundary – The absorbed dose in the cavity is deposited entirely by dT the charged particles crossing it • dx - mass collision stopping c • Additional assumptions: power, evaluated at energy T – Existence of CPE • Assuming continuous – Absence of bremsstrahlung generation across the interface – No backscattering 1 Bragg-Gray Theory Bragg-Gray Theory For differential energy distribution T, average mass For charge Q (of either sign) produced in gas