Reksten Et Al.2020 Nutrient Composition.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa 457: 1–8 (2004) ISSN 1175-5326 (Print Edition) ZOOTAXA 457 Copyright © 2004 Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 457: 1–8 (2004) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 457 Copyright © 2004 Magnolia Press ISSN 1175-5334 (online edition) Stephanostomum tantabiddii n. sp. (Digenea: Acanthocolpidae) from Carangoides fulvoguttatus (Forsskål, 1775) (Perciformes: Carangidae) from Ningaloo Reef, Western Australia RODNEY A. BRAY1 & THOMAS H. CRIBB2 1Department of Zoology, The Natural History Museum, Cromwell Road, London SW7 5BD, UK, email: [email protected] 2Centre for Marine Studies and Department of Microbiology and Parasitology, The University of Queensland, Brisbane, Queensland 4074, Australia, email: [email protected] Abstract A new species, Stephanostomum tantabiddii n. sp., is described from the yellowspotted trevally Carangoides fulvoguttatus from Ningaloo Reef, Western Australia. It has 38–45 circum-oral spines and the vitellarium reaches to no less than 17% of the hindbody length from the ventral sucker. It differs from other species of Stephanostomum with these characteristics by various combinations of the ventral hiatus of the circum-oral spine rows, the relatively long pars prostatica and short ejacu- latory duct, the elongate body and the wide gaps between the gonads. Key words: Stephanostomum tantabiddii n. sp., Acanthocolpidae, Digenea, Carangoides fulvogut- tatus, Carangidae, Perciformes, Ningaloo Coral Reef, Western Australia Introduction Members of the large genus Stephanostomum Looss, 1899 parasitise a wide variety of teleost hosts, but a significant proportion of them are found in fishes of the perciform fam- ily Carangidae. This family includes many large pelagic piscivores. The second intermedi- ate hosts of Stephanostomum are almost always found to be fishes and, as piscivores, carangids are therefore particularly susceptible to infection (Pérez-Urbiola & Martínez- Díaz, 2001). -

MARKET FISHES of INDONESIA Market Fishes
MARKET FISHES OF INDONESIA market fishes Market fishes indonesiaof of Indonesia 3 This bilingual, full-colour identification William T. White guide is the result of a joint collaborative 3 Peter R. Last project between Indonesia and Australia 3 Dharmadi and is an essential reference for fish 3 Ria Faizah scientists, fisheries officers, fishers, 3 Umi Chodrijah consumers and enthusiasts. 3 Budi Iskandar Prisantoso This is the first detailed guide to the bony 3 John J. Pogonoski fish species that are caught and marketed 3 Melody Puckridge in Indonesia. The bilingual layout contains information on identifying features, size, 3 Stephen J.M. Blaber distribution and habitat of 873 bony fish species recorded during intensive surveys of fish landing sites and markets. 155 market fishes indonesiaof jenis-jenis ikan indonesiadi 3 William T. White 3 Peter R. Last 3 Dharmadi 3 Ria Faizah 3 Umi Chodrijah 3 Budi Iskandar Prisantoso 3 John J. Pogonoski 3 Melody Puckridge 3 Stephen J.M. Blaber The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. ACIAR operates as part of Australia’s international development cooperation program, with a mission to achieve more productive and sustainable agricultural systems, for the benefit of developing countries and Australia. It commissions collaborative research between Australian and developing-country researchers in areas where Australia has special research competence. It also administers Australia’s contribution to the International Agricultural Research Centres. Where trade names are used, this constitutes neither endorsement of nor discrimination against any product by ACIAR. ACIAR MONOGRAPH SERIES This series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR’s research and development objectives. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

The Vertical Distribution of Fish on Two Offshore Oil Platforms
The vertical distribution of fish on two offshore oil platforms Thomas Tothill 21530074 Supervisors: Prof. Jessica Meeuwig1 Prof. Richard Hobbs2 1 Marine Future Lab, School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia 2 School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia Marine Ecology Progress Series This thesis is submitted in partial fulfillment of the requirements for a Master of Biological Sciences, specialising in Marine Biology BIOL556 Research Dissertation School of Biological Sciences Faculty of Science The University of Western Australia May 2019 Word Count: 8752 1 Abstract Many offshore oil and gas platforms around the globe are reaching their end-of-life and will require decommissioning in the next few decades. Australian legislation stipulates complete removal of obsolete platforms, however in situ decommissioning is currently under consideration. Knowledge on the ecology of offshore platforms in Australia is limited and the subsequent consequences of decommissioning remain poorly understood. Remotely operated vehicle (ROV) video is often collected during standard industry operations and may provide insight into the marine life associating with offshore platforms, however the utility of this video for scientific purposes remains unclear. Archival ROV video surveys of the Wandoo oil platforms on Australia’s North West Shelf were tested for its utility and found that the imagery was limited in scientific value due to the haphazard method of collection. It is recommended that future surveys conduct standardised transects in high definition video at constant speeds and orientations. Based on a subset of the usable ROV video, the influence of depth and structural complexity on taxonomic richness, abundance, biomass and assemblage structure of fish populations was assessed on the Wandoo oil platforms. -

Nematode Parasites of Four Species of Carangoides (Osteichthyes: Carangidae) in New Caledonian Waters, with a Description of Philometra Dispar N
Nematode parasites of four species of Carangoides (Osteichthyes: Carangidae) in New Caledonian waters, with a description of Philometra dispar n. sp. (Philometridae) František Moravec, Delphine Gey, Jean-Lou Justine To cite this version: František Moravec, Delphine Gey, Jean-Lou Justine. Nematode parasites of four species of Carangoides (Osteichthyes: Carangidae) in New Caledonian waters, with a description of Philometra dispar n. sp. (Philometridae). Parasite, EDP Sciences, 2016, 23, pp.40. 10.1051/parasite/2016049. hal-01399891 HAL Id: hal-01399891 https://hal.sorbonne-universite.fr/hal-01399891 Submitted on 21 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Parasite 2016, 23,40 Ó F. Moravec et al., published by EDP Sciences, 2016 DOI: 10.1051/parasite/2016049 urn:lsid:zoobank.org:pub:C2F6A05A-66AC-4ED1-82D7-F503BD34A943 Available online at: www.parasite-journal.org RESEARCH ARTICLE OPEN ACCESS Nematode parasites of four species of Carangoides (Osteichthyes: Carangidae) -

Marine Ecology Progress Series 530:223
The following supplement accompanies the article Economic incentives and overfishing: a bioeconomic vulnerability index William W. L. Cheung*, U. Rashid Sumaila *Corresponding author: [email protected] Marine Ecology Progress Series 530: 223–232 (2015) Supplement Table S1. Country level discount rate used in the analysis Country/Territory Discount rate (%) Albania 13.4 Algeria 8.0 Amer Samoa 11.9 Andaman Is 10.0 Angola 35.0 Anguilla 10.0 Antigua Barb 10.9 Argentina 8.6 Aruba 11.3 Ascension Is 10.0 Australia 6.5 Azores Is 7.0 Bahamas 5.3 Bahrain 8.1 Baker Howland Is 7.0 Bangladesh 15.1 Barbados 9.7 Belgium 3.8 Belize 14.3 Benin 10.0 Bermuda 7.0 Bosnia Herzg 10.0 Bouvet Is 7.0 Br Ind Oc Tr 7.0 Br Virgin Is 10.0 Brazil 50.0 Brunei Darsm 10.0 Country/Territory Discount rate (%) Bulgaria 9.2 Cambodia 16.9 Cameroon 16.0 Canada 8.0 Canary Is 7.0 Cape Verde 12.3 Cayman Is 7.0 Channel Is 7.0 Chile 7.8 China Main 5.9 Christmas I. 10.0 Clipperton Is 7.0 Cocos Is 10.0 Colombia 14.3 Comoros 10.8 Congo Dem Rep 16.0 Congo Rep 16.0 Cook Is. 10.0 Costa Rica 19.9 Cote d'Ivoire 10.0 Croatia 10.0 Crozet Is 7.0 Cuba 10.0 Cyprus 6.8 Denmark 7.0 Desventuradas Is 10.0 Djibouti 11.2 Dominica 9.5 Dominican Rp 19.8 East Timor 10.0 Easter Is 10.0 Ecuador 9.4 Egypt 12.8 El Salvador 10.0 Eq Guinea 16.0 Eritrea 10.0 Estonia 10.0 Faeroe Is 7.0 Falkland Is 7.0 Fiji 6.2 Finland 7.0 Fr Guiana 10.0 Fr Moz Ch Is 10.0 Country/Territory Discount rate (%) Fr Polynesia 10.0 France 4.0 Gabon 16.0 Galapagos Is 10.0 Gambia 30.9 Gaza Strip 10.0 Georgia 20.3 Germany (Baltic) 7.0 Germany (North Sea) 7.0 Ghana 10.0 Gibraltar 7.0 Greece 7.0 Greenland 7.0 Grenada 9.9 Guadeloupe 10.0 Guam 7.0 Guatemala 12.9 Guinea 10.0 GuineaBissau 10.0 Guyana 14.6 Haiti 43.8 Heard Is 7.0 Honduras 17.6 Hong Kong 7.4 Iceland 17.3 India 11.7 Indonesia 16.0 Iran 15.0 Iraq 14.1 Ireland 2.7 Isle of Man 7.0 Israel 6.9 Italy 5.8 Jamaica 17.5 Jan Mayen 7.0 Japan (Pacific Coast) 10.0 Japan (Sea of Japan) 10.0 Jarvis Is 10.0 Johnston I. -

Nematode Parasites of Four Species of Carangoides (Osteichthyes: Carangidae) in New Caledonian Waters, with a Description of Philometra Dispar N
Parasite 2016, 23,40 Ó F. Moravec et al., published by EDP Sciences, 2016 DOI: 10.1051/parasite/2016049 urn:lsid:zoobank.org:pub:C2F6A05A-66AC-4ED1-82D7-F503BD34A943 Available online at: www.parasite-journal.org RESEARCH ARTICLE OPEN ACCESS Nematode parasites of four species of Carangoides (Osteichthyes: Carangidae) in New Caledonian waters, with a description of Philometra dispar n. sp. (Philometridae) František Moravec1,*, Delphine Gey2, and Jean-Lou Justine3 1 Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branišovská 31, 370 05 Cˇ eské Budeˇjovice, Czech Republic 2 Service de Systématique moléculaire, UMS 2700 CNRS, Muséum National d’Histoire Naturelle, Sorbonne Universités, CP 26, 43 rue Cuvier, 75231 Paris cedex 05, France 3 ISYEB, Institut Systématique, Évolution, Biodiversité, UMR7205 CNRS, EPHE, MNHN, UPMC, Muséum National d’Histoire Naturelle, Sorbonne Universités, CP51, 57 rue Cuvier, 75231 Paris cedex 05, France Received 10 August 2016, Accepted 28 August 2016, Published online 12 September 2016 Abstract – Parasitological examination of marine perciform fishes belonging to four species of Carangoides, i.e. C. chrysophrys, C. dinema, C. fulvoguttatus and C. hedlandensis (Carangidae), from off New Caledonia revealed the presence of nematodes. The identification of carangids was confirmed by barcoding of the COI gene. The eight nematode species found were: Capillariidae gen. sp. (females), Cucullanus bulbosus (Lane, 1916) (male and females), Hysterothylacium sp. third-stage larvae, Raphidascaris (Ichthyascaris) sp. (female and larvae), Terranova sp. third- stage larvae, Philometra dispar n. sp. (male), Camallanus carangis Olsen, 1954 (females) and Johnstonmawsonia sp. (female). The new species P. dispar from the abdominal cavity of C. -
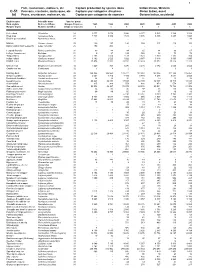
Fish, Crustaceans, Molluscs, Etc Capture
1 Fish, crustaceans, molluscs, etc Capture production by species items Indian Ocean, Western C-51 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Océan Indien, ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Océano Indico, occidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 1998 1999 2000 2001 2002 2003 2004 Nombre inglés Nombre científico Grupo de especies t t t t t t t Kelee shad Hilsa kelee 24 3 077 3 076 3 896 4 277 5 525 2 306 3 208 Hilsa shad Tenualosa ilisha 24 7 747 6 482 4 545 3 846 6 146 8 257 4 024 Bloch's gizzard shad Nematalosa nasus 24 ... ... ... ... ... ... 93 Milkfish Chanos chanos 25 139 137 125 154 117 113 101 Barramundi(=Giant seaperch) Lates calcarifer 25 196 204 - - - - - Leopard flounder Bothus pantherinus 31 90 84 84 65 84 96 67 Lefteye flounders nei Bothidae 31 9 27 129 125 88 73 78 Tonguefishes Cynoglossidae 31 2 158 2 066 2 137 1 923 1 989 1 383 984 Indian halibut Psettodes erumei 31 3 192 2 520 3 022 2 957 3 118 3 365 4 095 Flatfishes nei Pleuronectiformes 31 17 474 12 851 24 720 11 489 15 573 15 924 11 325 Unicorn cod Bregmaceros mcclellandi 32 1 449 743 1 470 2 435 2 372 2 643 2 624 Gadiformes nei Gadiformes 32 7 5 2 1 - 1 3 Bombay-duck Harpadon nehereus 33 144 865 146 663 133 221 141 082 100 366 101 936 116 452 Greater lizardfish Saurida tumbil 33 6 064 3 710 3 485 3 894 4 247 3 634 2 655 Brushtooth lizardfish Saurida undosquamis 33 34 20 30 32 20 11 18 Lizardfishes nei Synodontidae 33 20 221 20 871 17 894 12 433 12 447 10 715 13 833 Giant catfish Arius thalassinus 33 315 309 554 480 470 574 597 Sea catfishes nei Ariidae 33 99 932 98 467 78 375 89 308 89 941 78 788 81 972 Sabre squirrelfish Sargocentron spiniferum 33 34 53 46 97 96 135 114 Flathead grey mullet Mugil cephalus 33 69 89 60 34 37 40 46 Klunzinger's mullet Liza klunzingeri 33 .. -

National Report on the Fish Stocks and Habitats of Regional, Global
United Nations UNEP/GEF South China Sea Global Environment Environment Programme Project Facility NATIONAL REPORT on The Fish Stocks and Habitats of Regional, Global, and Transboundary Significance in the South China Sea THAILAND Mr. Pirochana Saikliang Focal Point for Fisheries Chumphon Marine Fisheries Research and Development Center 408 Moo 8, Paknum Sub-District, Muang District, Chumphon 86120, Thailand NATIONAL REPORT ON FISHERIES – THAILAND Table of Contents 1. MARINE FISHERIES DEVELOPMENT........................................................................................2 / 1.1 OVERVIEW OF THE FISHERIES SECTOR ...................................................................................2 1.1.1 Total catch by fishing area, port of landing or province (by species/species group).7 1.1.2 Fishing effort by gear (no. of fishing days, or no. of boats) .......................................7 1.1.2.1 Trawl ...........................................................................................................10 1.1.2.2 Purse seine/ring net....................................................................................10 1.1.2.3 Gill net.........................................................................................................12 1.1.2.4 Other gears.................................................................................................12 1.1.3 Economic value of catch..........................................................................................14 1.1.4 Importance of the fisheries sector -

And Their Fisheries In
SMALL PELAGIC RESOURCES AND THEIR FISHERIES IN THE ASIA-PACIFIC REGION ASIA-PACIFIC FISHERY COMMISSION FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS REGIONAL OFFICE FOR ASIA AND THE PACIFIC RAP Publication 1997/31 SMALL PELAGIC RESOURCES AND THEIR FISHERIES IN THE ASIA-PACIFIC REGION Proceedings of the First Session of the APFIC Worldng Party on Marine Fisheries Bangkok, Thailand, 13-16 May 1997 Asia-Pacific Fishery Commission Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific Bangkok, Thailand, 1997 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of itsauthorities, or concerning the delinutation of its frontiers or boundaries. Opinions expressed in this publication are those of the author(s) alone and do not imply any opinion whatsoever on the part of FAO and APFIC. NOTICE OF COPYRIGHT The copyright in this publication is vested in the Food and Agriculture Organization of the United Nations. This publication may not be reproduced, in whole or in part, by any method or process, without written permission from the copyright holder. Applications for such permission with a statement of the purpose and extent of the reproduction desired should be made through and addressed to the Senior Fishery Officer, FAO Regional Office for Asia and the Pacific, Maliwan Mansion, Phra Athit Road, Bangkok 10200, Thailand. ii PREPARATION OF THIS DOCUMENT This Proceedings consists of the Conclusion and Recommendations of and the papers presented at the First Session of the APFIC Working Party on Marine Fisheries held in Bangkok, Thailand, from 13 to 16 May 1997. -

Western Indian Ocean JOURNAL of Marine Science
Western Indian Ocean JOURNAL OF Marine Science Volume 18 | Issue 2 | Jul – Dec 2019 | ISSN: 0856-860X Chief Editor José Paula Western Indian Ocean JOURNAL OF Marine Science Chief Editor José Paula | Faculty of Sciences of University of Lisbon, Portugal Copy Editor Timothy Andrew Editorial Board Lena GIPPERTH Aviti MMOCHI Sweden Tanzania Serge ANDREFOUËT Johan GROENEVELD France Cosmas MUNGA South Africa Kenya Ranjeet BHAGOOLI Issufo HALO Mauritius South Africa/Mozambique Nyawira MUTHIGA Kenya Salomão BANDEIRA Christina HICKS Mozambique Australia/UK Brent NEWMAN Betsy Anne BEYMER-FARRIS Johnson KITHEKA South Africa USA/Norway Kenya Jan ROBINSON Jared BOSIRE Kassim KULINDWA Seycheles Kenya Tanzania Sérgio ROSENDO Atanásio BRITO Thierry LAVITRA Portugal Mozambique Madagascar Louis CELLIERS Blandina LUGENDO Melita SAMOILYS Kenya South Africa Tanzania Pascale CHABANET Joseph MAINA Max TROELL France Australia Sweden Published biannually Aims and scope: The Western Indian Ocean Journal of Marine Science provides an avenue for the wide dissem- ination of high quality research generated in the Western Indian Ocean (WIO) region, in particular on the sustainable use of coastal and marine resources. This is central to the goal of supporting and promoting sustainable coastal development in the region, as well as contributing to the global base of marine science. The journal publishes original research articles dealing with all aspects of marine science and coastal manage- ment. Topics include, but are not limited to: theoretical studies, oceanography, marine biology and ecology, fisheries, recovery and restoration processes, legal and institutional frameworks, and interactions/relationships between humans and the coastal and marine environment. In addition, Western Indian Ocean Journal of Marine Science features state-of-the-art review articles and short communications. -

National Report on the Fish Stocks and Habitats of Regional, Global
United Nations UNEP/GEF South China Sea Global Environment Environment Programme Project Facility NATIONAL REPORT on The Fish Stocks and Habitats of Regional, Global, and Transboundary Significance in the South China Sea CAMBODIA Mr. Ing Try Focal Point for Fisheries Fisheries Administration, Ministry of Agriculture, Forestry and Fisheries 186 Norodom Blvd. P.O. Box 582, Phnom Penh, Cambodia NATIONAL REPORT ON FISHERIES - CAMBODIA Table of Contents 1. BACKGROUND ............................................................................................................................1 1.1 OVERVIEW OF CAMBODIA’S FISHERIES SECTOR ......................................................................1 1.1.1 Total catch by fishing area, port of landing or province (by species/species group, 1990 onwards)................................................................................................1 1.1.2 Fishing effort by gear (number of fishing days/number of boats)..............................4 1.1.2.1 Trawl (Khmer name Uon Ohs)......................................................................5 1.1.2.2 Purse seine/ring net (Khmer name Uon Tith)...............................................6 1.1.2.3 Gill net (Khmer name Mong Paehk) .............................................................7 1.1.2.4 Other (push nets, trolling, hand line, long line, trap)...................................10 1.1.3 Economic value of catch (estimated or actual)........................................................12 1.1.4 Importance of the fisheries