The Association Between Socioeconomic Status and Pandemic Influenza: Systematic Review and Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

London's Swine Flu Pandemic
Central and North West London NHS Foundation Trust Executive Office Tel: 020 3214 5760 Fax: 020 3214 5761 4 February 2010 Sarah Hurcombe Assistant Scrutiny Manager Post Point 10 City Hall The Queen's Walk London SE1 2AA Dear Ms Hurcombe, I am responding to your request for feedback in relation to London's response to Swine Flu. !asked my Director of Nursing Practice, who is our Infection Control lead, to consider the questions you asked and he has provided me with the following comments: 1. Communication was a bit of an issue at first but this improved rapidly in time. I generally found that staff at NHS London were doing their best to advise where they could. Teleconferencing was a big help and saved a lot of travel time. One final point is that there wasn't any panic at any time when it was declared to be a pandemic. 2. At some of the meetings members of NHS London could become rather defensive to constructive comments. They appeared to take these as personal attacks. A more open debate on the challenges we were all facing would have been helpful. I hope that the above comments are useful. We look forward to viewing others views and feedback when published on your web-site. Yours sincerely, Claire Murdoch Chief Executive Trust Headquarters, Greater London House, Hampstead Road, London NWl 7QY Tel: 020 3214 5700 Fax: 020 3214 5701 www.cnwi.nhs.uk Dear Sarah, Further to the letter from James Cleverly, Chair of Health and Public Services Committee, and in answer to the questions posed: The Ealing Hospital NHS Trust has yet to complete its local debrief, but in general we believe we responded well to the challenge. -

Secret Assassins
.. “BIRD FLU” HOAX EXPOSED A. True Ott, PhD –4/5/06 Exactly 30 years ago, in 1976, National Headlines Proclaimed: “Swine Flu Pandemic Poised to Exceed 1918 Death Toll”. The national news media swept America away in an unprecedented fear-based, propaganda campaign to promote what is now recognized as a tremendous money-making hoax designed to enrich large pharmaceutical houses by selling billions of dollars worth of “Swine Flu Vaccinations” to an ignorant public. What stopped the massive scam? Three factors – 1. A few honest congressmen blew the whistle on the conspirators, 2. A few honest microbiologists and medical doctors also went public with the TRUTH, and 3. a precedent-setting series of lawsuits against Rockefeller’s “Big Five” pharmaceutical/vaccine manufacturing houses were victorious; many recipients of the “vaccine”experienced permanent paralysis and even death –all of this caused by the FDA approved and so-called “Safe, Well Tested Vaccine.” Today, alarmingly, THE EXACT SAME SCRIPT PLAYED IN 1976 is once again unfolding on the World Stage. Many of the “players” are the same. The goals of the conspirators are somewhat different however. Money does NOT appear to be the key issue today –POWER, MANIPULATION AND FASCIST CONTROL OF THE AMERICAN PEOPLE are apparently today the key issues. The Horror Story does not need to be re-written. All one has to do is read and internalize the work honest scientists published in 1976, and the history of the 1918 pandemic. The TRUTH still is TRUTH today, 30 years later. I would personally submit, the stakes are infinitely greater, however. -

EMERGING BIOLOGICAL THREATS This Page Intentionally Left Blank EMERGING BIOLOGICAL THREATS
EMERGING BIOLOGICAL THREATS This page intentionally left blank EMERGING BIOLOGICAL THREATS A Reference Guide JOAN R. CALLAHAN GREENWOOD PRESS An Imprint of ABC-CLIO, LLC Copyright 2010 by Joan R. Callahan All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except for the inclusion of brief quotations in a review, without prior permission in writing from the publisher. Library of Congress Cataloging-in-Publication Data Callahan, Joan R. Emerging biological threats : a reference guide / Joan R. Callahan. p. ; cm. Includes bibliographical references and index. ISBN 978-0-313-37209-4 (alk. paper) 1. Emerging infectious diseases. 2. Health risk assessment. 3. Food security. I. Title. [DNLM: 1. Disease Outbreaks—prevention & control. 2. Food Contamination—prevention & control. 3. Hazardous Substances—adverse effects. 4. Public Health—history. WA 105 C156e 2010] RA643.C263 2010 362.196'9—dc22 2009046252 14 13 12 11 10 12345 This book is also available on the World Wide Web as an eBook. Visit www.abc-clio.com for details. ISBN: 978-0-313-37209-4 EISBN: 978-0-313-37210-0 ABC-CLIO, LLC 130 Cremona Drive, P.O. Box 1911 Santa Barbara, California 93116-1911 This book is printed on acid-free paper Manufactured in the United States of America Dedicated to my great-grandfather MICHAEL FOLEY (1849–1897) Born during the Irish Famine Died from an experimental tuberculosis treatment This page intentionally left blank Contents Preface xi 1. Introduction 1 Public Health: A Short History 2 Koch and His Postulates 2 Hazard, Threat, and Risk 2 Outbreaks, Epidemics, and Pandemics 6 What Is Popular Culture? 6 More Definitions 6 So How Bad Is It? 6 2. -

(SARS-Cov-2, 2019-Coronavirus). J Mycol Mycological Sci 2020, 3(1): 000123
Open Access Journal of Mycology & Mycological Sciences ISSN: 2689-7822 MEDWIN PUBLISHERS Committed to Create Value for Researchers The Historical/Evolutionary Cause and Possible Treatment of Pandemic COVID-19 (SARS-CoV-2, 2019-Coronavirus) Niknamian S* Associate Professor of Medicine, John D Dingell VA Medical Center and Wayne State University, Review Article USA Volume 3 Issue 1 Received Date: May 02, 2020 *Corresponding author: Sorush Niknamian, Chief, Division of Infectious Diseases, John D Published Date: June 15, 2020 Dingell VA Medical Center, Associate Professor of Medicine, Wayne State University Detroit, MI DOI: 10.23880/oajmms-16000123 USA, Tel: 313-576-3057; Fax: 313-576-1242; Email: [email protected] Abstract Background: A virus is a small infectious agent that replicates only inside the living cells of an organism. Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity in a way virus. Some viruses especially smallpox, throughout history, has killed between 300-500 million people in its 12,000- analogous to sexual reproduction. Influenza (Including (COVID-19), is an infectious disease caused by an influenza year existence. As modern humans increased in numbers, new infectious diseases emerged, including SARS-CoV-2. We have two groups of virus, RNA and DNA viruses. The most brutal viruses are RNA ones like COVID-19 Sars-CoV-2. Introduction: Coronaviruses are a group of viruses that cause diseases in mammals and birds. In humans, coronaviruses cause respiratory tract infections that are typically mild, such as some cases of the common cold and COVID-19. -

Annals of Medical & Surgical Case Reports
1 Volume 02; Issue 03 ISSN: 2652-4414 Annals of Medical & Surgical Case Reports Research Article Niknamian S and Zaminpira S. Ann Med &Surg Case Rep: AMSCR-100050 The Historical/Evolutionary Cause and Possible Treatment of Pandemic Covid-19 (Sars-Cov-2, 2019-Coronavirus) Niknamian S1* and Zaminpira S2 1Department of Military Medicine,American Military University and Liberty University, USA 2Department of Phycology and Psychiatry,American Beirut University, Beirut, Lebanon *Corresponding author: Sorush Niknamian, Department of Military Medicine, American Military University and Liberty University, USA, Tel: 989121939806; Email: [email protected] Citation: Niknamian S and Zaminpira S (2020) The Historical/Evolutionary Cause and Possible Treatment of Pandemic Covid-19 (Sars-Cov-2, 2019-Coronavirus). Ann Med &Surg Case Rep: AMSCR-100050 Received date: 11 April, 2020; Accepted date: 20 April, 2020; Published date: 27 April, 2020 Abstract Background: A virus is a small infectious agent that replicates only inside the living cells of an organism. Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity in a way analogous to sexual reproduction.Influenza (Including (COVID-19), is an infectious disease caused by an influenza virus. Some viruses especially smallpox, throughout history, has killed between 300-500 million people in its 12,000-year existence.As modern humans increased in numbers, new infectious diseases emerged, including SARS-CoV-2. We have two groups of virus, RNA and DNA viruses. The most brutal viruses are RNA ones like COVID-19 (Sars-CoV-2). -

The Monster at Our Door Also by Mike Davis
The Monster at Our Door Also by Mike Davis Prisoners of the American Dream: Politics and Economy in the History of the US Working Class City of Quartz: Excavating the Future in Los Angeles Ecology of Fear: Los Angeles and the Imagination of Disaster Magical Urbanism: Latinos Reinvent the US City Late Victorian Holocausts: El Nino Famines and the Making of the Third World Under the Perfect Sun:The San Diego Tourists Never See (with Jim Miller and Kelly Mayhew) Dead Cities and Other Tales The Monster at Our Door The Global Threat of Avian Flu Mike Davis THE NEW PRESS NEW YORK LONDON © 2005 by Mike Davis All rights reserved. No part of this book may be reproduced, in any form, without written permission from the publisher. Requests for permission to reproduce selections from this book should be mailed to: Permissions Department, The New Press, 38 Greene Street, New York, NY 10013 Published in the United States by The New Press, New York, 2005 Distributed by W. W. Norton & Company, Inc., New York LIBRARY OF CONGRESS CATALOGING-IN-PUBLICATION DATA Davis, Mike, 1946- The monster at our door : the global threat o f avian flu / Mike Davis, p. cm. Includes bibliographical references and index. ISBN 1-59558-011-5 (he.) 1. Avian influenza—Popular works. I. Tide RA644.I6D387 2005 636.5'0896203— dc22 2005043853 The New Press was established in 1990 as a not-for-profit alternative to the large, commercial publishing houses currently dominating the book publishing industry. The New Press operates in the public interest rather than for private gain, and is committed to publishing, in innovative ways, works of educational, cultural, and community value that are often deemed insufficiently profitable. -

Swine Flu Expose by Eleanora I
Eleanora McBean Ph.D., N.D. http://www.whale.to/vaccine/sf.html Quotes "TYPHOID MARY" WAS NEVER A CARRIER by Eleanora I. McBean, Ph.D., N.D. Books [1977] Vaccination, The Silent Killer----Honorof, Ida, and McBean. [1977] Swine Flu Expose by Eleanora I. McBean, Ph.D., N.D. [1957 CLASSIC] THE POISONED NEEDLE by Eleanor McBean [1957] The Hidden Dangers In Polio Vaccine (Chapter 10 of Poisoned Needle) by Eleanor McBean [Home] Swine Flu Expose a book by Eleanora I. McBean, Ph.D., N.D. Chapter 1 Swine Flu--Another Medically-made Epidemic Preface $5,000,000 law suit filed against flu promoters for causing paralysis Swine flu vaccine tests prove unsafe No swine flu epidemic All vaccines are poison Ford the puppet Vaccines can trigger heart attack 80% of all medical doctors are unfit Drugs cannot cure Vaccine promoters try to dodge the blame A few congressmen blast the swine flu hoax How to punish the culprits Law suits against the vaccine promoters American medical Association has been outlawed New Anti-trust suit against the chiropractors 2,000,000 doses of defective swine flu vaccine condemned Many fake findings by scientists seen Swine flu promoters tried to con the swine raisers President Coolidge unswayed by medical pressure God give us men What our leaders should have checked on before throwing us to the killer (vaccine) sharks Question that should have been answered before considering any vaccination drive Death count may never be known Who pockets the money? Compulsory army vaccinations are unconstitutional How will our new President Carter handle the swine flu swindle? Fluoridated tooth paste dangerous A better campaign PREFACE This booklet on Swine Flu hazards and fakery is part of a larger book on vaccination, titled, VACCINATION CONDEMNED BY COMPETENT DOCTORS. -
List of Epidemics
List of epidemics This is a list of the biggest known epidemics (including pandemics) caused by an infectious disease. Widespread non-communicable diseases such as cardiovascular disease and cancer are not included. An epidemic is the rapid spread of disease to a large number of people in a given population within a short period of time. For example, in meningococcal infections, an attack rate in excess of 15 cases per 100,000 people for two consecutive weeks is considered an epidemic.[1] 17th-century German "plague panel" depicting the triumph of death. Panels of this kind were placed on the walls of houses to warn against the plague. A plague epidemic raged in Augsburg, Bavaria between 1632 and 1635 Emergency hospital during Spanish Influenza epidemic, Camp Funston, Kansas (1918-20) Death toll Event Date Location Disease Ref. (estimate) Babylon, or Babirus of the Persians, Sanskrit scholars found Influenza 1200 BC Central Asia, records of a disease Unknown [2] epidemic Mesopotamia resembling the Flu and Southern Asia Unknown, possibly Plague of 429–426 Greece, Libya, typhus, typhoid fever or 75,000–100,000 [3][4][5][6] Athens BC Egypt, Ethiopia viral hemorrhagic fever Greece 412 BC (Northern Unknown, possibly 412 BC Unknown [7] epidemic Greece, Roman influenza Republic) 165–180 Unknown, possibly Antonine Plague (possibly Roman Empire 5–10 million [8] smallpox up to 190) Plague of Unknown, possibly 250–266 Europe 1 million+ [9][10] Cyprian smallpox 25–100 million (40– Plague of Europe and Bubonic plague (beginning 541–542 50% of population [11][12][13] Justinian West Asia of First plague pandemic) of Europe) Rome, Roman Plague Bubonic plague (part of 590 Byzantine Unknown [14] of 590 First plague pandemic) Empire Plague of Bubonic plague (part of 627–628 Mesopotamia Unknown [15] Sheroe First plague pandemic) Bubonic plague (part of Plague of 664 664–689 British Isles Unknown [16] First plague pandemic) Byzantine Plague of 698– Empire, West Bubonic plague (part of 698–701 Unknown [17] 701 Asia, Syria, First plague pandemic) Mesopotamia 735–737 2 million (approx. -

Process of Respiration, the Relationship of Air to Diseases of the the Impact on Air Quality of Human Activities and the Effect
DOCUMENT EESUME ED 174 411 SE 027 980 TITLE Biomedical Science, Unit I: Respiration in Health and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases; Introductory Chemistry; and Air Pollution. Student Text. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPCNS AGENCY National Science Foundation, hashington, D.C. PUB DATE 75 NOTE 274p.; For related documents, see SE 027 978-999 and SE 028 510-516; Not available in hard copy due to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIPTCRS Anatomy; Chemistry; Environment; *Health; *Health Education; Higher Education; Medical Education; Pathology; Physics; *Physiology; *Science Education; Secondary Education IDENTIFIERS *Respiration ABSTRACT This student text deals with the human respiratory system and its relation to the environment. Topics include the process of respiration, the relationship of air to diseases of the respiratory system, the chemical and physical properties of gases, the impact on air quality of human activities and the effect of this air pollution on health. Superimposed on the lessons in scientific principles throughout the text is a continuing case study of a man's life span using respiratory health as the concept under analysis in this context. (RE) *********************************************************************** Reproductions supplied by EDRS are the best that can be made from the original document. *********************************************************************** -

View Presentation
THE NATIONAL ECONOMY DISRUPTIVE EPIDEMICS AND THE U.S. ECONOMY U.S. Annual GDP Growth and Epidemic Deaths 1910 - 2020 20% Polio (1916) 7,000 deaths Polio (1946-52) London Flu (1972-73) 7,710 deaths 1,072 deaths 15% Swine SARS (2002-03) Flu Asian Flu (1957-58) 0 deaths (2009-10) 10% 116,000 deaths 7,000 deaths Hong Kong Flu (1968-69) 100,000 deaths 5% 0% Ebola (2014-15) -5% 2 deaths COVID-19 (2020-) -10% 220,000+ deaths Spanish Flu (1918-19) 675,000 deaths -15% 1910 1915 1920 1925 1930 1935 1940 1945 1950 1955 1960 1965 1970 1975 1980 1985 1990 1995 2000 2005 2010 2015 2020* *estimated Source: Bureau of Economic Analysis, CDC, University of Groningen, Delta Associates; October 2020. QUARTERLY GDP PERCENT CHANGE United States 10% 20-Year Average = 2.0% 5% 0% -5% -10% -15% Q2 2020 = -31.4% -20% -25% -30% -35% 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Note: Seasonally adjusted at annual rates; change in chained 2012 dollars. Source: Bureau of Economic Analysis, Delta Associates; October 2020. INITIAL UNEMPLOYMENT CLAIMS United States | Four-Week Moving Average in Millions Great Expansion Period COVID-19 Recession 6.0 Recession Peak (Week of 03/28/20) = 5,790,250 5.5 Millions 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Peak (Week of 3/28/09) = 659,250 (Week of 10/10/20) = 866,250 0.5 0.0 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Note: Data are seasonally adjusted. -
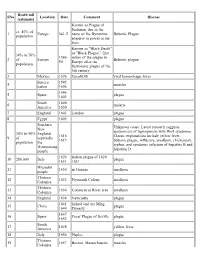
List of Epidemics
Death toll SNo Location Date Comment Disease (estimate) Known as Plague of Justinian, due to the ca. 40% of 1 Europe 541-2 name of the Byzantine Bubonic Plague population emperor in power at the time. Known as "Black Death" or "Black Plague," first 30% to 70% 1346- return of the plague to 2 of Europe Bubonic plague 50 Europe after the population Justinianic plague of the 6th century. 3 Mexico 1576 Cocolitztli viral hemorrhagic fever Seneca 1592– 4 measles nation 1596 1596– 5 Spain plague 1602 South 1600– 6 malaria America 1650 7 England 1603 London plague 8 Egypt 1609 plague Southern Unknown cause. Latest research suggests New epidemic(s) of leptospirosis with Weil syndrome. 30% to 90% England, 1616– Classic explanations include yellow fever, 9 of especially 1619 bubonic plague, influenza, smallpox, chickenpox, population the typhus, and syndemic infection of hepatitis B and Wampanoag hepatitis D. people 1629– Italian plague of 1629– 10 280,000 Italy plague 1631 1631 Wyandot 11 1630 in Ontario smallpox people Thirteen 12 1633 Plymouth Colony smallpox Colonies Thirteen 13 1634 Connecticut River area smallpox Colonies 14 England 1636 Newcastle plague 1641– helped end the Ming 15 China plague 1644 Dynasty 1647– 16 Spain Great Plague of Seville plague 1652 South 17 1648 yellow fever America 18 Italy 1656 Naples plague Thirteen 19 1657 Boston, Massachusetts measles Colonies 1663– 20 24,148 Netherlands Amsterdam plague 1664 1665– 21 100,000 England Great Plague of London plague 1666 22 40,000 France 1668 plague 1676– 23 Spain plague 1685 24 -

London NHS 111 IUC and GPOOH Influenza
London NHS 111 Integrated Urgent Care, resident registered GP and GP Out of Hours Influenza Outbreak Response during a COVID-19 Pandemic Standard Operating Procedure (SOP) for Care Providers NHS England and NHS Improvement London Integrated Urgent Care Provider Influenza outbreak SOP Authors: Version 1.8 • Dr May Cahill (GP Clinical Lead- Enhanced Health in Care Homes) • Ezra Kanyimo (Enhanced Health in Care Homes Lead) • Tyler Smith (Healthy London Partnership) Contributors: • Dr Catherine Heffernan (Principal Advisor for Commissioning Early Years, Immunisations and Vaccination Services) • Laura Cook (NHS England and NHS Improvement (London Region) • Jemma Sharples (Quality Manager) • Sam Perkins (Public Health England (PHE) / London region) • Dr Caroline Chill (Health Innovation Network) • Maria Rodrigues (Senior Programme Manager-Transforming Primary Care) • Deirdre Malone (NHS North Central London CCG) • Jas Khambh (NHS London Procurement Partnership) Status: First draft Version issue date: 29 September 2020 SOP Category Operational Document management Revision History Version Date Summary of changes 1 24 July 2020 First draft meeting comments 2 Reviewers Reviewer Name Title/responsibility Date This document Version must be reviewed by the Briony Sloper Out-Of-Hospital Cell Lead 23/09/20 following people: Samantha Perkins Public Health England (PHE) / 14/08/20 London region John Light Integrated Urgent Care (IUC) 21/08/20 Mobilisation Lead Dr Catherine Heffernan Immunisation Lead (Principal 14/08/20 Advisor for Commissioning Early