Stick 'N' Peel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exceptional Fossil Preservation During CO2 Greenhouse Crises? Gregory J
Palaeogeography, Palaeoclimatology, Palaeoecology 307 (2011) 59–74 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Exceptional fossil preservation during CO2 greenhouse crises? Gregory J. Retallack Department of Geological Sciences, University of Oregon, Eugene, Oregon 97403, USA article info abstract Article history: Exceptional fossil preservation may require not only exceptional places, but exceptional times, as demonstrated Received 27 October 2010 here by two distinct types of analysis. First, irregular stratigraphic spacing of horizons yielding articulated Triassic Received in revised form 19 April 2011 fishes and Cambrian trilobites is highly correlated in sequences in different parts of the world, as if there were Accepted 21 April 2011 short temporal intervals of exceptional preservation globally. Second, compilations of ages of well-dated fossil Available online 30 April 2011 localities show spikes of abundance which coincide with stage boundaries, mass extinctions, oceanic anoxic events, carbon isotope anomalies, spikes of high atmospheric carbon dioxide, and transient warm-wet Keywords: Lagerstatten paleoclimates. Exceptional fossil preservation may have been promoted during unusual times, comparable with fi Fossil preservation the present: CO2 greenhouse crises of expanding marine dead zones, oceanic acidi cation, coral bleaching, Trilobite wetland eutrophication, sea level rise, ice-cap melting, and biotic invasions. Fish © 2011 Elsevier B.V. All rights reserved. Carbon dioxide Greenhouse 1. Introduction Zeigler, 1992), sperm (Nishida et al., 2003), nuclei (Gould, 1971)and starch granules (Baxter, 1964). Taphonomic studies of such fossils have Commercial fossil collectors continue to produce beautifully pre- emphasized special places where fossils are exceptionally preserved pared, fully articulated, complex fossils of scientific(Simmons et al., (Martin, 1999; Bottjer et al., 2002). -
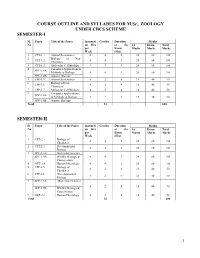
COURSE OUTLINE and SYLLABUS for M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I
COURSE OUTLINE AND SYLLABUS FOR M.Sc., ZOOLOGY UNDER CBCS SCHEME SEMESTER-I Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-1.1 Animal Systematics 4 4 3 20 80 100 2 Biology of Non- CPT-1.2 4 4 3 20 80 100 Chordates 3 CPT-1.3 Molecular Cell Biology 4 4 3 20 80 100 4 Computer Applications & SPT-1.4A Methods in Biology 4 4 3 20 80 100 SPT-1.4B Aquatic Biology 5 CPP-1.5 Animal Systematics 4 2 4 10 40 50 6 Biology of Non- CPP-1.6 4 2 4 10 40 50 Chordates 7 CPP-1.7 Molecular Cell Biology 4 2 4 10 40 50 8 Computer Applications SPP-1.8A & Methods in Biology 4 2 4 10 40 50 SPP-1.8B Aquatic Biology Total 24 600 SEMESTER-II Sl. Paper Title of the Paper Instructi Credits Duration Marks No on Hrs of the IA Exam Total per Exam Marks Marks Marks Week (Hrs) 1 CPT-2.1 Biology of 4 4 3 20 80 100 Chordates 2 CPT-2.2 Developmental 4 4 3 20 80 100 Biology 3 SPT-2.3A Molecular Genetics SPT-2.3B Wildlife Biology & 4 4 3 20 80 100 Conservation 4 OET-2.4 Human Physiology 4 4 3 20 80 100 5 CPP-2.5 Biology of 4 2 4 10 40 50 Chordates 6 CPP-2.6 Developmental 4 2 4 10 40 50 Biology 7 SPP-2.7A Molecular Genetics 4 2 4 10 40 50 SPP-2.7B Wildlife Biology & Conservation 8 OEP-2.8 Human Physiology 4 2 4 10 40 50 Total 24 600 1 SEMESTER-III Sl. -

ROCEK, Z. and WUTTKE, M. (2010) Amphibia of Enspel (Late
Palaeobio Palaeoenv (2010) 90:321–340 DOI 10.1007/s12549-010-0042-0 ORIGINAL PAPER Amphibia of Enspel (Late Oligocene, Germany) ZbyněkRoček & Michael Wuttke Received: 23 April 2010 /Revised: 9 July 2010 /Accepted: 12 August 2010 /Published online: 29 September 2010 # Senckenberg Gesellschaft für Naturforschung and Springer 2010 Abstract Amphibia from the Late Oligocene (MP 28) One specimen is a large premetamorphic tadpole (no locality Enspel, Germany are represented by two caudates: rudimentary limbs) with a total body length of 147 mm. a hyperossified salamandrid Chelotriton paradoxus and an Anatomically, it can be equally assigned to Pelobates or to indeterminate salamandrid different from Chelotriton in Eopelobates; the second possibility was excluded only on proportions of vertebral column. Anurans are represented the basis of absence of adult Eopelobates in this locality. by two forms of the genus Palaeobatrachus, one of which is nearly as large as P. gigas (now synonymized with P. Keywords Enspel . Oligocene . Salamandridae . grandipes). Pelobates cf. decheni, represented in this Chelotriton . Anura . Palaeobatrachus . Pelobates . Rana locality by three nearly complete adult skeletons and a large number of tadpoles, is the earliest record for the Abbreviation genus. Compared with later representatives of the genus, it DP FNSP Department of Palaeontology Faculty of does not yet possess specializations for burrowing. Ranidae Natural Sciences, Prague are represented by two rather fragmentary and incomplete skeletons referred to as Rana sp. A comparatively large series of tadpoles was assigned to the Pelobatidae on the basis of tripartite frontoparietal complex. Most of them are Introduction premetamorphic larvae, and a few older ones are post- metamorphic, but they do not exceed Gossner stage 42. -

Larval Development in Oligocene Palaeobatrachid Frogs
Larval development in Oligocene palaeobatrachid frogs ZBYNĚK ROČEK Roček, Z. 2003. Larval development in Oligocene palaeobatrachid frogs. Acta Palaeontologica Polonica 48 (4): 595–607. A detailed account of the development of skeletal and some soft−tissue structures is based on 171 fossil tadpoles and meta− morphosing froglets of Palaeobatrachus sp. from the Late Oligocene of the Czech Republic (locality Bechlejovice). Their exceptionally good preservation resulted from fossilization in diatomites. The fossil developmental series was com− pared with normal development of the contemporary anuran Xenopus laevis (Pipidae) represented by cleared and stained (alizarin/toluidin−blue) whole−mount specimens. The comparison revealed that in spite of differences in the sequence of ossification and its timing (e.g., ossification of the otic capsules and ribs was retarded in Xenopus whereas dermal ossifi− cation was retarded in Palaeobatrachus), in the number of free ribs, and in composition of the sacral region (the synsacrum in Palaeobatrachus involves two posterior presacrals, whereas there is a single sacral in Xenopus), both gen− era were similar in great number of anatomical features that appear during development. The most important difference is the shape of vertebral centrum (procoelous in Palaeobatrachus, opisthocoelous in all Pipidae) which is formed in com− paratively early developmental stages. A view that could result from anatomical comparisons is that Palaeobatrachus could be derived from the Pipidae, but this is doubtful due to biostratigraphic and palaeogeographic discrepancies. The earliest palaeobatrachids were recorded from the Late Cretaceous of Europe but pipids could not invade northern conti− nents after the Early Cretaceous when the Tethys Sea prevented interchanges of anuran faunas. -

Frontoparietal Bone in Extinct Palaeobatrachidae (Anura): Its Variation and Taxonomic Value
THE ANATOMICAL RECORD 298:1848–1863 (2015) Frontoparietal Bone in Extinct Palaeobatrachidae (Anura): Its Variation and Taxonomic Value ZBYNEK ROCEK, 1* RENAUD BOISTEL,2 NICOLAS LENOIR,3 ARNAUD MAZURIER,4 STEPHANIE E. PIERCE,5 JEAN-CLAUDE RAGE,6 SERGEI V. SMIRNOV,7 ACHIM H. SCHWERMANN,8 XAVIER VALENTIN,2 9 10 11 MARTON VENCZEL, MICHAEL WUTTKE, AND TOMA S ZIKMUND 1Department of Palaeobiology, Geological Institute, Academy of Sciences of the Czech Republic, Prague, Czech Republic 2Institut International de Paleoprimatologie et de Paleontologie Humaine, UMR 7262 CNRS, Universite de Poitiers, Poitiers, France 3Multiscale Group, Laboratoire Navier, UMR8205-CNRS/ENPC/IFSTTAR/Universite Paris-Est, Champs-sur-Marne, France 4Institut de Chimie des Milieux et Materiaux de Poitiers, UMR 7285 Universitede Poitiers, UFR SFA, Poitiers, France 5Department of Organismic and Evolutionary Biology and Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 6Sorbonne Universites—CR2P—MNHN, CNRS, UPMC-Paris 6, Museum National d’Histoire Naturelle, Paris, France 7Laboratory of Evolutionary Morphology, a.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia 8Steinmann-Institut fur€ Geologie, Mineralogie und Palaontologie,€ Universitat€ Bonn, Bonn, Germany 9 T; arii Cris¸urilor Museum, Oradea, Romania 10Department of Archaeology, General Department of Cultural Heritage Rhineland Palatinate, Section Geological History of the Earth, Mainz, Germany 11X-Ray Micro CT and Nano CT Research Group, CEITEC—Central European Institute of Technology, Brno University of Technology, Brno, Czech Republic ABSTRACT Palaeobatrachidae are extinct frogs from Europe closely related to the Gondwanan Pipidae, which includes Xenopus. Their frontoparietal is a distinctive skeletal element which has served as a basis for establishing the genus Albionbatrachus. -

Retallack 2011 Lagerstatten
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Palaeogeography, Palaeoclimatology, Palaeoecology 307 (2011) 59–74 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Exceptional fossil preservation during CO2 greenhouse crises? Gregory J. Retallack Department of Geological Sciences, University of Oregon, Eugene, Oregon 97403, USA article info abstract Article history: Exceptional fossil preservation may require not only exceptional places, but exceptional times, as demonstrated Received 27 October 2010 here by two distinct types of analysis. First, irregular stratigraphic spacing of horizons yielding articulated Triassic Received in revised form 19 April 2011 fishes and Cambrian trilobites is highly correlated in sequences in different parts of the world, as if there were Accepted 21 April 2011 short temporal intervals of exceptional preservation globally. Second, compilations of ages of well-dated fossil Available online 30 April 2011 localities show spikes of abundance which coincide with stage boundaries, mass extinctions, oceanic anoxic events, carbon isotope anomalies, spikes of high atmospheric carbon dioxide, and transient warm-wet Keywords: Lagerstatten paleoclimates. -

Icp Publications 2006-2021
ICP PUBLICATIONS 2006-2021 Last updated: 1 September 2021 In press and published online _______________________________________________________________________ SCI papers (indexed in JCR) 1. Abella, J., Martín-Perea, D. M., Valenciano, A., Hontecillas, D., Montoya, P., & Morales, J. (2021, published online). Coprolites in natural traps: direct evidence of bone eating carnivorans from the Late Miocene site of Batallones-3 (Madrid, Spain). Lethaia. https://doi.org/10.1111/let.12438 2. Agustí, J., Espresate, J., & Piñero, P. (2020, published in press). Dental variation in the endemic dormouse Hypnomys Bate 1918 and its implications for the palaeogeographic evolution of the Balearic Islands (Western Mediterranean) during the late Neogene-Quaternary. Historical Biology. https://doi.org/10.1080/08912963.2020.1852557 3. Alba, D. M., Robles, J. M., Valenciano, A., Abella, J., & Casanovas-Vilar, I. (2021, published online). A new species of Eomellivora from the latest Aragonian of Abocador de Can Mata (NE Iberian Peninsula). Historical Biology. https://doi.org/10.1080/08912963.2021.1943380 4. Arias-Martorell, J., Zeininger, A., & Kivell, T. L. (in press). Trabecular structure of the elbow reveals divergence in knuckle-walking biomechanical strategies of African apes. Evolution. 5. Bouchet, F., Urciuoli, A., Beaudet, A., Pina, M., Moyà-Solà, S., & Alba, D. M. (in press). Comparative anatomy of the carotid canal in the Miocene small-bodied catarrhine Pliobates cataloniae. Journal of Human Evolution. 6. Caballero, Ó., Montoya, P., Crespo, V. D., Morales, J., & Abella, J. (2020, published online). The autopodial skeleton of Paracamelus aguirrei (Morales 1984) (Tylopoda, Mammalia) from the late Miocene site of Venta del Moro (Valencia, Spain). Journal of Iberian Geology. -

The Present Paper Describes for the First Time in Detail the Herpeto Fauna from the Locality of W~Ze II
Acta Palaeontologica Polonica Vol. 29, No . 3-4 pp.209-226 VVarszavva, 1984 MARIAN MLYNARSKI, ZBIGNIEVV SZYNDLAR, RICHARD \.ESTES and BORJA SANCHlz AMPHIBIANS AND REPTILES FROM THE PLIOCENE LOCALITY OF WEiZE II NEAR DZIALOSZYN (POLAND) . MLYNARSKI, M., SZYNDLAR, Z. , ESTES, R. and SANCHIZ, B.: Amphibians and reptiles from the Pliocene locality of VV<;ze II near Dzialoszyn (Poland). Acta Palaeont. Polonica, 29, 3-4, 209-226, 1985 (1984). , The following forms have been recognized in the Upper Pliocene fauna from the karstic deposits near Dzialoszyn: Mtoproteus wezet sp, n. (Proteidae), pataeoba trachus sp. and p!tobatrachus cr. tanghae (Palaeobatrachidae), Petobates fuscus (Pelobatidae), Bufo bUfo (Bufonidae), Rana sp, (Ranidae), Emys orbtcutarts an ttgua (Emydidae), Ophlsaurus pannontcus and Anguts ct. fraglUs (Anguidae), La certa cr. v trtdts and Lacerta sp, (Lacertidae), Etaphe paratongtsstma and Natrtx ct. tongtvertebrata (Colubridae). The majority of the recognized taxa, including living forms (e.g, Bufo tniio, Emys orblcutarts, Anguts fraglUs) as well as extinct ones (Pltobatrachus tanqhae. Natrtx tongtvertebrata), were widespread in the Euro pean Neogene lind ·(e ven tuall y) Pleistocene. Key W 0 r d s : Amphibia, Reptilia, Pliocene, Poland. Marian ilnynarski and Zbtgniew Szyndtar. Zaklad zootoglt Systematycznej t 00 Awtadczatnej, Potska Akademia Nauk, S!awkowska 17, 31-016 Krak6w, Potand. Richard Estes, Department of zootogy, CoUege Of Sctence, San otego, CA 92182-0063, USA. Borja Sanchtz, Museo Nactonat de Ctenctas Naturates (C.S.I.C.), Castettana, 80, Madrid-G, Spa tn. INTRODUCTION The present paper describes for the first time in detail the herpeto fauna from the locality of W~ze II. This fossil site was discovered by Sulimski (1962) and named W~ze II in order to distinguish it from the nearby locality W~Ze 1. -

(Urodela: Batrachosauroididae) of Europe Davit Vasilyan1,2* & Vadym Yanenko3
www.nature.com/scientificreports OPEN The last Palaeoproteus (Urodela: Batrachosauroididae) of Europe Davit Vasilyan1,2* & Vadym Yanenko3 The Batrachosauroididae are an enigmatic group of salamanders known from the Cretaceous and Tertiary of North America and Europe. In Europe, the family is known only by two species of the genus Palaeoproteus. The genus has limited distribution in Western and Central Europe. In the present paper, we describe a new species, Palaeoproteus miocenicus, from the early late Miocene (11–9 Ma) of Austria and Ukraine, representing the youngest record of the family Batrachosauroididae from the Neogene of Europe. The new species difers from the Paleogene representatives of the genus by 12 characters, including large body size, the long anterior extension of the Meckelian groove and the size and shape of the odontoid process on the dentary. The µCT scanning of bones of the new species revealed novel features (e.g. anterior extension of Meckelian groove, interconnected network of canals and small cavities in atlas) observable only in this species. P. miocenicus inhabited aquatic environments, which existed under wet climatic conditions with mean annual precipitation higher than 900 mm. The new species expands the temporal range of the genus by at least 30 million years and enlarges the palaeogeographic distribution of the genus into Eastern Europe. Te present-day European salamander fauna is represented by the Salamandridae, Plethodontidae, Proteidae and Hynobiidae families1,2. Besides these families, the Cenozoic record of European salamanders includes also the extant family of Cryptobranchidae3, as well as several enigmatic salamanders such as Batrachosauroididae4, an ambistomoid5, Seminobatrachus6, Bergmannia7, Geyeriella8 and Wolterstorfella8. -

Palaeobatrachid Frog from the Late Miocene of Northern Caucasus, Russia
Palaeontologia Electronica palaeo-electronica.org Palaeobatrachid frog from the late Miocene of Northern Caucasus, Russia Elena V. Syromyatnikova ABSTRACT Disarticulated remains of Palaeobatrachus sp. (Palaeobatrachidae) from the late Miocene (early Turolian, MN 11; about 8–7.5 m.y.) of Volchaya Balka and Gaverdovsky localities (Northern Caucasus, Russia) are described. Palaeobatrachus from Volchaya Balka and Gaverdovsky differs from other species of the genus by having four premax- illary teeth. It shows an intermediate dentition between pre-Pliocene palaeobatrachids with five or more premaxillary teeth and Pleistocene congeners with two or three pre- maxillary teeth. The variations in the frontoparietals and synsacra may indicate that two species of Palaeobatrachus co-existed in Volchaya Balka and Gaverdovsky. The wide contribution of the transverse processes of V7+8+9 in the sacral wings of Palaeobatra- chus from Volchaya Balka and Gaverdovsky shows that palaeobatrachids probably reached an extreme degree of aquatic adaptation as early as the late Miocene or even earlier. The Caucasian Palaeobatrachus partly fills the late Miocene gap (MN 10–13) in palaeobatrachid distribution and extends the Miocene range of this family to eastern- most Europe (European Russia). Elena V. Syromyatnikova. A.A. Borissiak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya ul., 123, Moscow, 117997 Russia; [email protected]; [email protected] and Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034 Russia. Keywords: Anura; Palaeobatrachidae; Palaeobatrachus; late Miocene, Caucasus; Russia Submission: 24 February 2018 Acceptance: 23 July 2018 INTRODUCTION 2016) to the middle Pleistocene (e.g., Ratnikov, 1993, 1997, 2002; Wuttke et al., 2012). The Asian Palaeobatrachidae Cope, 1865, are common records include Palaeobatrachus sp. -

Newsletter Number 58
The Palaeontology Newsletter Contents 58 Association Business 2 News 8 Association Meetings 10 Meeting Reports 14 Mysterious Fossils 25 From our correspondents Inferring Developmental Mode 26 Foiling vertebrate inversion 32 PalaeoBioSuperstar 40 Palaeo-math 101: Regression 4 44 Obituary: Ehrhard Voigt 54 Progressive Palaeontology 2005 57 Future meetings of other bodies 58 Book Reviews 72 Palaeontology vol 48 parts 1 & 2 92–93 Discounts for PalAss members 94 Reminder: The deadline for copy for Issue no 59 is 20th May 2005 On the Web: <http://www.palass.org/> Newsletter 58 2 Newsletter 58 3 Association Business the compilation of fossil collections, and their care and conservation, to published studies in recognised journals. Nominations should comprise a short statement (up to one page of A4) outlining the candidate’s principal achievements. Members putting forward candidates should Nominations for Council also be prepared, if requested, to write an illustrated profile in support of their nominee. The deadline for nominations is 1st September 2005. The award comprises a cash prize plus a At the AGM in December 2005, the following vacancies will occur on Council: framed scroll, and is usually presented at the Annual meeting. Vice-President Sylvester-Bradley Award Newsletter Editor three handling editors Awards are made to assist palaeontological research (travel, visits to museums, fieldwork etc.), one Ordinary member with each award having a maximum value of £1,000. Preference is given to applications for a single purpose (rather than top-ups of other grant applications) and no definite age limit is Nominations are now invited for these posts. Please note that each candidate must be proposed applied, although some preference may be given to younger applicants or those at the start by at least two members of the Association and that any individual may not propose more than of their careers. -

SHORT NOTES the Extinct Anuran
SHORT NOTES 35 HERPETOLOGICAL JOURNAL, Vol. 6, pp. 35-36 (1996) A P ALAEOBATRACHID ANURAN ILIUM FROM THE BRITISH EOCENE J. ALAN HOLMAN Michigan State University Museum, East Lansing, Michigan 48824-1045, USA The extinct anuran family Palaeobatrachidae Cope extends from the Late Jurassic to the Plio-Pleistocene (Meszoely et al., 1984). The most abundant genus, Palaeobatrachus, occupied a broad adaptive zone in FIG. I. Palaeobatrachus sp. ilia. A, leftili um (Michigan space and in time, occurring in both the Old and New State University Museum Vertebrate Paleontology Number World, and existing from Cretaceous through Miocene 1630) from the Totland Bay Locality, Totland Bay Member, times. The ecological niche of Palaeobatrachus is Headon Hill Formation, Late Eocene, Isle of Wight, thought to have been similar to that of the modern England. B, left ilium from the Late Paleocene of Cernay, highly aquatic pipid frog Xe nopus. Palaeobatrachids France, drawn from a photograph in Vergnaud-Grazzini & have been reported from the British Isles only from a Hoffs tetter (1972). C, left ilium from the Rijksuniversiteit Utrecht Collection, Early Oligocene of Belgium, after Estes single Late Eocene locality at Headon Hill on the Isle & Sanchiz (1982). Abbreviations are: g = glutaeus tubercle, of Wight (Rage Ford, 1980; Meszoely et al., 1984), & i = iliofibularis-iliofemoralis attachment. The 3 mm line and fromanother Late Eocene locality on the sea at the applies to all three drawings. nearby Hordle Cliff, Hampshire, (Milner et al., 1982). These fossilremains did not include ilia. IA) was collected by David Harrison in 1991 at the Isle British palaeobatrachid material in Rage & Ford of Wight Totland Bay Locality, a stratigraphic equiva (1980) and Milner et al.