The Reston Chlorofluorocarbon Laboratory CFC Sampling Method - Bottles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glass Container Styles
Glass Container Styles Glass Bottles & Jars Boston Round Bottles Boston Round Bottles are general use bottles that are perfect for liquids, product storage, and field or plant sampling projects. They feature a round body, rounded shoulders and narrow screw neck opening. These environmentally sensitive bottles help eliminate waste and help to ensure product integrity for long term storage. Clear / Flint Boston Round Bottles offers maximum visibility and sample integrity. Amber & Cobalt Blue Boston Round Bottles protect contents from UV rays and are ideal for light sensitive products. Composite Test Jars Clear / Flint Composite Test Jars are clear wide-mouth straight sided jars that are ideal for sampling and provide maximum content visibility. These bottles are designed without shoulders for maximum storage capacity. French Square Bottles Clear / Flint French Square Bottles provide maximum content visibility. The space saving design saves on shelf and storage space. The wide mouth opening is ideal for mixing, storing and sampling. Graduated Medium Round Bottles Bottle Beakers® also known as Graduated Medium Rounds are excellent for use with biological and pathological specimens, but can also be used for storing industrial laboratory chemicals and reagents. These clear / flint bottles are designed with a slight shoulder for easy pouring and handling. Graduated in ml and ounces. Mix, measure, and store in the same container. Media Bottles Media Bottles are manufactured from PYREX® borosilicate glass for chemical and thermal resistance and can be used for storage as well as mixing and sampling. Regular Media Bottles have permanent white enamel graduations and marking spots. PYREXPlus® Media Bottles have a protective PVC coating helps prevent glass from shattering and reduces spills. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout -

Boston Round Glass Bottles
Boston Round Glass Bottles Finneran Products Certifi ed For ScienceTM - Available in clear or amber glass - Choice of closure and liner - Available in standard, precleaned or precleaned/certifi ed in accordance with recommended E.P.A. Protocol CLASS 1: (Standard) Containers are assembled with liner and closure without washing treatment. CLASS 2: (Precleaned) Containers are processed according to EPA recommended wash procedures. Washed with laboratory grade biodegradable, non-phosphate detergent, rinsed with ASTM Type I De-ionized water, oven dried, and assembled in a contaminate-free environment. Th e exterior of each case is labeled with a lot number. Each case contains a copy of wash “Standard Operating Procedure.” CLASS 3: (Certifi ed) Containers are processed according to EPA recommended wash procedures. Washed with laboratory grade biodegradable, non-phosphate detergent, rinsed with ASTM Type I De-ionized water, oven dried and assembled in a contaminate-free environment. Th e exterior of each case is labeled with a lot number. Each case contains a “Certifi cate of Analysis.” An independent laboratory is used to certify the container’s cleanliness, assuring impartial results. Standard Assembled with White Polypropylene Closure, .015” PTFE Lined Clear Glass Boston Round Bottles - Class 1 Cat. No. Description Qty 9-170 1 oz, 30mL Clear Boston Round Bottle, 20-400mm Th read, White Closure, PTFE Lined 24 Pack 9-171 2 oz, 60mL Clear Boston Round Bottle, 20-400mm Th read, White Closure, PTFE Lined 24 Pack 9-172 4 oz, 125mL Clear Boston Round Bottle, 22-400mm Th read, White Closure, PTFE Lined 24 Pack 9-173 8 oz, 250mL Clear Boston Round Bottle, 24-400mm Th read, White Closure, PTFE Lined 12 Pack 9-174 16 oz, 500mL Clear Boston Round Bottle, 28-400mm Th read, White Closure, PTFE Lined 12 Pack 9-175 32 oz, 1000mL Clear Boston Round Bottle, 33-400mm Th read, White Closure, PTFE Lined 12 Pack Amber Glass Boston Round Bottles - Class 1 Cat. -

Qorpak™ Laboratory Container & Supply Catalog
Laboratory Container & Supply Catalog TABLE OF CONTENTS Page # Page # Page # New Products Buckets/Pails Vials, Plastic HDPE Square Bottles . .3 Open Head HDPE Pails.................40 Hinged .............................57 Bags Snap Cap ...........................57 Zip Bags . .4 Carboys & Drums Carboys.............................41 Weigh Dishes . 58 Bottles, Glass Hedpaks® ...........................41 Dropper Bottles ......................12 Packos .............................42 Technical Information Feature Bottles.....................5 - 6 Winpaks® ...........................41 Conversion Chart .....................58 Jugs ...............................14 How to Select the Right Bottle & Closure..59 Narrow Mouth Bottles...............7 - 8 Cubitainers® .......................43 Plastic Resin Guide ...................37 Safety Coated Bottles .............15 - 17 Trademarks................. Back Cover Wide Mouth Bottles................9 - 13 Caps/Closures UN Ratings Guide.....................41 Closure Torque System ........43Hole Caps Bottles, Plastic 44 Dispensing Bottles....................36 Metal Caps..........................49 Jars............................35 - 36 Phenolic & Thermoset Caps.........44 - 47 Jugs ...........................38 - 39 Polypropylene Caps ...............47 - 49 Narrow Mouth Bottles.............30 - 31 Septa & Discs........................50 Did You Know? Spray Bottles ........................37 Specialty Dispensing Caps..........50 - 51 Wide Mouth Bottles...............32 - 34 • Qorpak has been a -

The Effect of Liquid Hot Filling Temperature on Blow-Molded HDPE Bottle Properties
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2008-12-04 The Effect of Liquid Hot Filling Temperature on Blow-Molded HDPE Bottle Properties Benjamin S. Hudson Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Manufacturing Commons BYU ScholarsArchive Citation Hudson, Benjamin S., "The Effect of Liquid Hot Filling Temperature on Blow-Molded HDPE Bottle Properties" (2008). Theses and Dissertations. 1624. https://scholarsarchive.byu.edu/etd/1624 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. THE EFFECT OF LIQUID HOT FILLING TEMPERATURE ON BLOW-MOLDED HDPE BOTTLE PROPERTIES by Benjamin S. Hudson A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science School of Technology Brigham Young University December 2008 BRIGHAM YOUNG UNIVERSITY GRADUATE COMMITTEE APPROVAL of a thesis submitted by Benjamin S. Hudson This thesis has been read by each member of the following graduate committee and by majority vote has been found to be satisfactory. Date Mike P. Miles, Chair Date A. Brent Strong Date Scott D. Grimshaw BRIGHAM YOUNG UNIVERSITY As chair of the candidate’s graduate committee, I have read the thesis of Benjamin S. Hudson in its final form and have found that (1) its format, citations, and bibliographical style are consistent and acceptable and fulfill university and department style requirements; (2) its illustrative materials including figures, tables, and charts are in place; and (3) the final manuscript is satisfactory to the graduate committee and is ready for submission to the university library. -

Product Catalogue Digital Version Home Brew Kit
PRODUCT CATALOGUE WWW.THEPLASTICBOTTLESCOMPANY.COM DIGITAL VERSION HOME BREW KIT Following a brainstorming session, we came up with the brilliant idea of putting together some of our great looking PET HUSKY bottles in a handy Vaping Home Brew kit. We talked to our vaping customers to find out what products they would like to see in the kit and we came up with what we think is a pretty comprehensive collection of bottles and accessories to get vapers sorted with their home mixing requirements. The Home Brew Kit is available in Clear bottle and Smokey bottle versions and includes: 4 x 60ml HUSKY short-fill bottles & twin-seal caps 4 x 120ml HUSKY short-fill bottles & twin-seal caps 8 x 10ml PET bottles, nibs & caps CLICK HERE 1 x 1L Jerry can with peli pump TO SEE HUSKYS ON 1 x 500ml Jerry can with peli pump Clear bottles Smokey bottles 2 01229 588877 • 01229 588468 Natural HDPE Bottles ..................4 Amber PET Bottles & Jar ..........16 Caps & Accessories ..........................5 Caps & Accessories ........................17 Natural HDPE Bottles ..................6 LDPE & MDPE Bottles ................18 Caps & Accessories ..........................7 Caps & Accessories ........................19 Black HDPE Bottles .......................8 PP, SAN & PET Jars .......................20 Caps & Accessories ..........................9 Linea & Cosmetic Jars................21 White HDPE Bottles ....................10 HUSKY Bottles & Caps ...............22 Caps & Accessories ........................11 Jerrycans .........................................23 -

Bottlesinsmall Case for Unlimitedapplications
BOTTLES KIMAX® media bottles are the perfect bottle for any application. The outstanding quality ensures a wide range of use, from long term storage and transporting to the most demanding applications in the pharmaceutical and food industries. Sturdy design and improved clarity allow contents and volume to be checked quickly, while temperature resistance makes the bottles ideal for autoclaving. Essential to every laboratory, KIMAX® media bottles are proven reliable for unlimited applications. We offer a wide variety of general purpose bottles in small case quantities or large bulk packs with a variety of closures. We also offer containers with or without caps attached for high use items or facilities with centralized stockrooms. Customization to meet your specific needs is simpler than ever, including pre-cleaning and barcoding. Trust DWK Life Sciences to be the exclusive source for all your laboratory glass needs. DWK Life Sciences 22 BOTTLES Clear Glass Boston Round / Amber Glass Boston Round Clear Glass Boston Round Bottles Amber Glass Boston Round Bottles Kimble® Clear Boston Rounds are made from Type III Kimble® Amber Boston Rounds are made from Type III soda-lime glass and have a narrow-mouth design. Clear soda-lime glass and have a narrow-mouth design. Amber bottles allow for viewing of contents. They come with a bottles protect light-sensitive contents. They come with a variety of caps and liner combinations and are designed variety of caps and liner combinations. They are designed to protect the quality of liquids and product storage. to protect contents from UV rays and are ideal for light- sensitive products. -

Velocity Scientific Solutions
Velocity Scientific Solutions ISO 9001:2008 Design, Engineering and Manufacturing Chromatography, Biotechnology and Environmental Products W: www.velocityscientific.com.au E: [email protected] P: 1300 855 315 Velocity Scientific Solutions Table of Contents Information Intro i History of Innovation ii General Conditions of Sale iii Technical Information iv Chromatography C1-C61 12x32mm Standard Opening Crimp Vial, 11mm Crimp Finish C1 12x32mm, Vista Vial™ C38 12x32mm Big Mouth Crimp Top Vials, 11mm Crimp Finish C4 15x45mm Snap Seal™ Vials, 13mm Crimp Finish C42 12x32mm Snap Ring™/Crimp Top Vials, 11mm Crimp Finish C8 15x45mm WISP™ Style Screw Thread Vials, 13-425mm Neck Finish C45 12x32mm Snap Seal™ Vials, 11mm Crimp Finish C13 15x45mm Shell Vials C48 12x32mm Standard Opening Screw Thread Vials, 8-425mm Neck Finish C18 8x40mm Shell Vials for Waters WISP™, 8mm Plug C50 12x32mm Large Opening R.A.M.™ Vials, 9mm Neck Finish C22 Dram Vials C53 12x32mm Big Mouth Screw Thread Vials, 10-425mm Finish C29 Headspace Vials C55 12x32mm Shell Vials C33 Injection Port GC Septa C57 12x32mm Versa Vials™, 9mm Opening C36 Capsule Holder™ C61 Biotechnology B1-B16 96-Well Multi-Tier™ Micro Plate System B1 Sealing Films for 96-Well Microplates B11 Aluminum 96-Well Micro Plate System B6 Sealing Films for Cell and Tissue Culture, ELISA, EIA, B12 and Similar Assays Snap Seal™ Vials and Snap Top Caps™ for 96-Well Multi-Tier™ B8 Micro Plate System Sealing Films for PCR and Cold Storage B13 Snap Seal™ Vials and Snap Top Caps™ for 96-Square Deep B9 Molded -

WHEATON Media Bottles
> 1 Proven Tools™ for Scientific Research Mission Contact Info WHEATON is a First-Tier Best In Class Global Supplier, a highly effective > USA & Canada ...................................... 800.225.1437 marketer and a product / service innovator serving the general laboratory, life science and diagnostics packaging segments. > International ......................................... 856.825.1100 > Worldwide Fax ...................................... 856.825.1368 Our Life’s work is founded in our unrelenting passion for Customer Satisfaction and Performance Improvement making us incredibly easy to do business with. > Website ..........................................www.wheaton.com Our Associates take pride in our product, our workplace and in performance. > Street ...................................... 1501 North 10th Street Please contact us and our friendly associates will be glad to assist you. > City / State / Zip.....................Millville, NJ 08332-2038 > Country ................................................................USA > Hours ................................ 8:00 a.m. to 5:00 p.m. EST Stephen R. Drozdow President, Chief Executive Officer > 2 Table of Contents Cell Culture Adherent Culture > Incubators * .....................................................................52 > Roller Apparatus * ............................................................51 > Roller Bottles ...................................................................51 > Vented Caps ....................................................................51 -
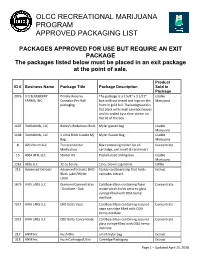
Olcc Recreational Marijuana Program Approved Packaging List
OLCC RECREATIONAL MARIJUANA PROGRAM APPROVED PACKAGING LIST PACKAGES APPROVED FOR USE BUT REQUIRE AN EXIT PACKAGE The packages listed below must be placed in an exit package at the point of sale. Product ID # Business Name Package Title Package Description Sold in Package 2076 3 D BLUEBERRY Private Reserve The package is a 2 5/8" x 3 1/12" Usable FARMS, INC. Cannabis Pre-Roll box with our brand and logo on the Marijuana packaging front in gold foil. The background is flat black with small cannabis leaves and its sealed by a clear sticker on the lid of the box. 1107 3Littlebirds, LLC Bobby's Bodacious Buds Mylar gusset bag Usable Marijuana 1108 3Littlebirds, LLC 3 Little Birds Usable Mj Mylar Gusset Bag Usable Bag Marijuana 8 420 Pharm LLC Transcendental Box containing holder for oil Concentrate Medication cartridge, cart inself & card insert 15 4964 BFH, LLC Starter Kit Pocket-sized sliding box. Usable Marijuana 1262 Ablis LLC 12 oz bottle 12 oz crown cap bottle Edible 213 Advanced Extracts Advanced Extracts BHO Sturdy cardboard slip that holds Extract Black Label/White cannabis extract. Label. 1679 AIRA LABS LLC Diamond Concentrates Cardboard box containing foam Concentrate - Distillate - Dab matte which holds secured glass syringe filled with ODA hemp distillate 1921 AIRA LABS LLC CBD Delta Vape Cardboard box containing secured Concentrate vape cartridge filled with ODA hemp distillate 1922 AIRA LABS LLC CBD Delta Concentrate Cardboard box containing secured Concentrate glass syringe filled with ODA hemp distillate 217 ANM Inc. Hush Bho small mylar bag Extract 218 ANM Inc. -

Thermo Scientific Nalgene Wide-Mouth Natural HDPE Bottle HDPE, PP Screw Closure Screw Closure
Thermo Scientifi c EnvironmentalThermo Scientifi Sample c Containers Environmental Sample Containers containers for water and soil sample collection that meet or exceed EPA requirements environmental sample containers | Two Great Names, One Leading Brand. Thermo Scientific™ Environmental Sample Containers have two decades of experience, quality performance and know-how behind them. Your two favorite brands and market leaders, EP Scientific and I-Chem come together, combining the best qualities and strengths of each to make one leading brand – Thermo Scientific. We offer a full line of environmental sampling containers processed to meet or exceed EPA requirements. You can trust Thermo Scientific Environmental Sample Containers to meet the highest quality standards you’ve come to trust through our leadership brands. Thermo Scientific Environmental Sample Containers are manufactured in our ISO 9001 manufacturing facilities in the US. All of our processes – from design to development to manufacturing – meet or exceed the requirements for quality as set forth by the International Standards Organization. In addition, we have developed Thermo Scientific Performance-Based Specifications, which guarantee our processed and certified products meet or exceed U.S. EPA analyte specifications as required in Section II.B of the current “Specifications and Guidance for Contaminant-Free Sample Containers” document. This document is available upon request by contacting our Technical Support Department at 1-800-550-4964. Specification criteria include volatiles, semi-volatiles, pesticides, PCBs and metals. 2 www.thermoscientifi c.com environmental sample packagingcontainers superior application performance www.thermoscientifi c.com 3 Environmental Sample Containers Table of Contents Thermo Scientific Products Page Thermo Scientific Products Page Clear VOA Vials with 0.125 in. -

C.L. Smith Solutions Catalog
UN Certifier Distributor Designer Manufacturer Your Partner in Packaging Solutions • HAZPlus® • CL SMITH CONTAINER • LYONS BLOW MOLDING MAKING YOUR LIFE EASIER www.clsmith.com www.hazplus.com 800.264.1202 866.596.6786 OUR CUSTOMER PROMISE We Are All In For You! Years of experience working with hundreds of companies like yours has taught us a very valuable lesson: To meet a challenge you have to own it. And what drives us as a company is the desire to do everything in our power to Make Your Life Easier! Manufacturer Reliability » Shorter Runs & Reduced Lead Times » Industry’s Highest Quality Standards Our Promise to You » Dedicated to Lean Manufacturing WE ARE HERE TO FUNCTION AS AN EXTENSION OF YOUR BUSINESS; AND » New, Standardized, State-of-the Art Equipment GIVE YOU MORE VALUE--NOT MORE TO MANAGE » Customized Enhancements, Styles and Sustainable Options • WE WILL OFFER A WIDE RANGE OF PRODUCTS AND INNOVATIVE OPTIONS • u Certifier Compliance n u WE WILL SHARE MARKET AND INDUSTRY INFORMATION » On-site n Performance Oriented Package Testing Facilityity TO KEEP YOU INFORMED » Turn-Key Services: Design, Test, Certify and Manufacturee • » Experts Available to Come On-Site to Review WE WILL OFFER COMPETITIVE PRICES THAT SAVE YOU MONEY; Regulatory Changes and Updates With You • » Custom Designs and Stock Packages Offered » Recertification Management Assures Proactive Compliancence WE WILL RESOLVE ISSUES QUICKLY AND TAKE ACCOUNTABILITY TO ENSURE YOUR SATISFACTION Designer Distinctive UN Certifier » Exclusive Molds and Bottle Designs Distributor » Collaboration to Differentiate Your Product Designer » 3D CAD Modeling and Rapid Prototyping Manufacturer » Quick Turnaround for Speed to Market Your Partner in Packaging Solutions • HAZPlus® • CL SMITH CONTAINER • LYONS BLOW MOLDING Distributor Dependability » Dedicated Personnel on Your Account » Buying Power Allows Competitive Pricing » Numerous Distribution Sites for Customer Stocking Programs » Just-In-Time Deliveries 2 OUR FAMILY OF SOLUTIONS C.L.