Read This Medication Guide Before You Start Taking Felbamate and Each Time You Get a Refill
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Pharmacokinetic Drug–Drug Interactions Among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients
International Journal of Molecular Sciences Review Pharmacokinetic Drug–Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients Marta Kara´zniewicz-Łada 1 , Anna K. Główka 2 , Aniceta A. Mikulska 1 and Franciszek K. Główka 1,* 1 Department of Physical Pharmacy and Pharmacokinetics, Poznan University of Medical Sciences, 60-781 Pozna´n,Poland; [email protected] (M.K.-Ł.); [email protected] (A.A.M.) 2 Department of Bromatology, Poznan University of Medical Sciences, 60-354 Pozna´n,Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-(0)61-854-64-37 Abstract: Anti-epileptic drugs (AEDs) are an important group of drugs of several generations, rang- ing from the oldest phenobarbital (1912) to the most recent cenobamate (2019). Cannabidiol (CBD) is increasingly used to treat epilepsy. The outbreak of the SARS-CoV-2 pandemic in 2019 created new challenges in the effective treatment of epilepsy in COVID-19 patients. The purpose of this review is to present data from the last few years on drug–drug interactions among of AEDs, as well as AEDs with other drugs, nutrients and food. Literature data was collected mainly in PubMed, as well as google base. The most important pharmacokinetic parameters of the chosen 29 AEDs, mechanism of action and clinical application, as well as their biotransformation, are presented. We pay a special attention to the new potential interactions of the applied first-generation AEDs (carba- Citation: Kara´zniewicz-Łada,M.; mazepine, oxcarbazepine, phenytoin, phenobarbital and primidone), on decreased concentration Główka, A.K.; Mikulska, A.A.; of some medications (atazanavir and remdesivir), or their compositions (darunavir/cobicistat and Główka, F.K. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

5 Clinical Pearls for Contraception and Preconception Counseling
EDITORIAL Women with epilepsy: 5 clinical pearls for contraception and preconception counseling For women with epilepsy, intrauterine devices are the optimal reversible contraceptive, and, preconception, the use of antiepileptic drugs with the lowest teratogenic potential should be considered Robert L. Barbieri, MD Editor in Chief, OBG MANAGEMENT Chair, Obstetrics and Gynecology Brigham and Women’s Hospital, Boston, Massachusetts Kate Macy Ladd Professor of Obstetrics, Gynecology and Reproductive Biology Harvard Medical School, Boston n 2015, 1.2% of the US population bazepine (Aptiom), felbamate (Fel- becoming pregnant while taking the was estimated to have active epi- batol), oxcarbazepine (Trileptal), oral contraceptive.6 Carbamazepine, Ilepsy.1 For neurologists, key goals perampanel (Fycompa), phenobarbi- a strong inducer of hepatic enzymes, in the treatment of epilepsy include: tal, phenytoin (Dilantin), primidone was the most frequently used AED in controlling seizures, minimizing (Mysoline), rufinamide (Banzel), this sample. adverse effects of antiepileptic drugs and topiramate (Topamax) (at dos- Many studies report that carba- (AEDs) and optimizing quality of ages >200 mg daily). According to mazepine accelerates the metabo- life. For obstetrician-gynecologists, Lexicomp, the following AEDs do not lisms of estrogen and progestins and women with epilepsy (WWE) have cause clinically significant changes reduces contraceptive efficacy. For unique contraceptive, preconcep- in hepatic enzymes that metabolize example, in one study 20 healthy tion, and obstetric needs that require steroid hormones: acetazolamide women were administered an ethi- highly specialized approaches to (Diamox), clonazepam (Klonopin), nyl estradiol (20 µg)-levonorgestrel care. Here, I highlight 5 care points ethosuximide (Zarontin), gabapentin (100 µg) contraceptive, and randomly that are important to keep in mind (Neurontin), lacosamide (Vimpat), assigned to either receive carbamaze- when counseling WWE. -

NMMC West Point CDM.Xlsx
Facility Description Price CC SWING BED RUGS CODE CC PRIVATE 1ST WEST 861 CC PRIVATE OBSTETRICS 861 CC BAKRI BALLOON FOR PP HEMR 661 CC BAKRI BALLOON FOR PP HEMR 661 CC RPR CMPX LID/N/L 2.6‐7.5 1914 CC RPR CMPX LID/N/ E/A 5CM/< 1053 CC RPR CMPX LID/N/L 2.6‐7.5 1914 CC RPR CMPX LID/N/ E/A 5CM/< 1053 CC PRIVATE ECU 494 CC IV THRPY FIRST HR DX 691 CC IV THRPY EA/ADD HR DX 190 CC IV THRPY ADD/SEQ TO 1 HR 205 CC IV THRPY CONCURRENT 122 CC IV THRPY FIRST HR HYDRTN 690 CC IV THRPY EA/ADD HR HYDRTN 134 CC INJ IV EA ADD DIFF MED 269 CC RECOVERY PER HOUR 280 CC MINOR PROCEDURE 1627 CC ARTERIAL LINE PLACEMENT 463 CC INJ EPIDURAL BLOOD PATCH 2850 CC INJ IV PUSH INITIAL 692 CC ADDITIONAL CPT CODE CC MED ICU ROOM CHARGE 1872 CC NURSERY 1200 CC CIRC TRAY 91 CC INIT HOSP CARE LV 1 P/DAY 205 CC INIT HOSP CARE LV 2 P/DAY 279 CC INIT HOSP CARE LV 3 P/DAY 412 CC SUBS HOSP CARE LV 1 P/DAY 81 CC SUBS HOSP CARE LV 2 P/DAY 147 CC SUBS HOSP CARE LV 3 P/DAY 212 CC HOSP DAY MGMT 30 MNT OR < 147 CC HOSP DAY MGMT > 30 MNTS 214 CC OBS CARE D/C DAY MGMT 155 CC INITL OBS CARE LV 1 P/DAY 197 CC INITL OBS CARE LV 2 P/DAY 239 CC INITL OBS CARE LV 3 P/DAY 362 CC SUBS OBS CARE LV 1 P/DAY 88 CC SUBS OBS CARE LV 2 P/DAY 145 CC SUBS OBS CARE LV 3 P/DAY 236 CC SNF D/C DAY MGMT 30 OR < 240 CC SNF D/C DAY MGMT >30 318 CC PERC REPLAC J TUBE W/O FL 1948 CC INITIAL NF CARE LEVEL 1 204 CC INITIAL NF CARE LEVEL 2 290 CC INITIAL NF CARE LEVEL 3 368 CC SUBSQENT NF CARE LEVEL 197 CC SUBSQENT NF CARE LEVEL 2 151 CC SUBSQENT NF CARE LEVEL 3 198 CC SUBSQENT NF CARE LEVEL 4 295 CC OBS OR I/P -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Status Epilepticus
Status Epilepticus By Aaron M. Cook, Pharm.D., BCPS, BCCCP Reviewed by Gretchen M. Brophy, Pharm.D., FCCP, BCPS; Matthew J. Korobey, Pharm.D. BCCCP; and You Min Sohn, Pharm.D., M.S., BCPS, BCCCP LEARNING OBJECTIVES 1. Evaluate factors that may affect treatment success in patients with status epilepticus. 2. Distinguish gaps in the literature related to optimal status epilepticus treatment. 3. Evaluate therapeutic strategies for super-refractory status epilepticus. 4. Assess the impact of timing of status epilepticus treatment initiation, and develop strategies to optimize effective treatment. DEFINITIONS OF STATUS EPILEPTICUS ABBREVIATIONS IN THIS CHAPTER Status epilepticus is defined as continuous seizure activity for EEG Electroencephalogram greater than 5 minutes or consecutive seizures without regaining GABA g-Aminobutyric acid consciousness over 5 minutes. Status epilepticus is common in ICH Intracerebral hemorrhage the epilepsy population and is often associated with acute, severe NMDA N-methyl-D-aspartate neurological injury or illness such as traumatic brain injury (TBI), TBI Traumatic brain injury intracerebral hemorrhage (ICH), meningitis, or pharmacologic toxic- Table of other common abbreviations. ity/withdrawal. Overall, the incidence of status epilepticus is about 12 per 100,000 individuals, a value that has increased 50% since the early 2000s (Dham 2014). Status epilepticus is often divided into “convulsive” status epilepticus (in which the patient has obvi- ous clinical manifestations of seizures, mental status impairment, or postictal focal neurological deficits) and “nonconvulsive” status epi- lepticus (in which the patient has no obvious clinical manifestations of seizure, but seizure activity is revealed on electroencephalogram [EEG]). Refractory status epilepticus is defined as status epilepticus that persists despite treatment with at least two antiepileptic drugs. -

Felbamate.Pdf
Core Safety Profile Active substance: Felbamate Pharmaceutical form(s)/strength: 400 and 600 mg, tablets 600mg/5ml, oral suspension P-RMS: FR/H/PSUR/0002/001 Date of FAR: 27.02.2013 4.2 Posology and method of administration /.../Felbamate should be used only under the supervision of a neurologist or a pediatrician with expertise in the treatment of epilepsy. LENNOX-GASTAUT SYNDROME Dosage in adults and adolescents 14 years and older: Adjunctive therapy with other antiepileptic agents : /.../felbamate administered in combination with carbamazepine, phenytoin, phenobarbital or valproic acid may increase the incidence of their characteristic adverse reactions (see section 4.5). Initiate /.../felbamate dosage at 600 mg to 1200 mg/day, administered in 2 or 3 divided doses. At onset of /.../felbamate therapy, reduce the dose of concomitant carbamazepine, phenytoin, phenobarbital, and/or valproic acid initially by 20% to 30%. /.../felbamate dosage may then be titrated in increments of 600 mg/day to 1200 mg/day at intervals of about one week to a maximum of 3600 mg/day administered in 3 or 4 divided doses. Dose adjustment of carbamazepine, phenytoin, phenobarbital, and valproic acid should be considered as /.../felbamate dose increases. However, interactions are dose-dependent and subject to individual patient variability. Therefore, all dose adjustments of concomitant antiepileptic medicines should be based not only on steady-state plasma concentrations but also on clinical observations. Pediatric dosage: children 4 to 11 years old and adolescents 12 to 14 years old Adjunctive therapy with other antiepileptic agents: /.../felbamate in combination with carbamazepine, phenytoin, phenobarbital, or valproic acid may increase the incidence of their characteristic adverse reactions (see section 4.5). -

Drug Class Review Newer Anticonvulsant Agents 28:12:92 Anticonvulsants, Other
Drug Class Review Newer Anticonvulsant Agents 28:12:92 Anticonvulsants, Other Brivaracetam (Briviact®) Clobazam (Onfi®) Eslicarbazepine (Aptiom®) Ezogabine (Potiga®) Felbamate (Felbatol®, others) Gabapentin (Neurontin®) Lacosamide (Vimpat®, others) Lamotrigine (Lamictal®, others) Levetiracetam (Keppra®, others) Oxcarbazepine (Trileptal®, Oxtellar XR®, others) Perampanel (Fycompa®) Pregabalin (Lyrica ®) Rufinamide (Banzel®) Tiagabine (Gabitril®) Topiramate (Topamax ®, Trokendi XR, Qudenxi XR, others) Vigabatrin (Sabril®) Zonisamide (Zonergan ®, others) Final Report June 2016 Review prepared by: Vicki Frydrych, Clinical Pharmacist University of Utah College of Pharmacy Copyright © 2016 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Introduction ....................................................................................................................... 1 Table 1: Comparison of Newer Anticonvulsant Agents ...................................... 2 Table 2: FDA-Approved Indications for Newer Anticonvulsant Agents ........... 16 Disease Overview ............................................................................................................ 17 Table 3: The International League Against Epilepsy Classification of Seizures ............................................................................................... 19 Table 4: Newer Antiepileptic Drugs Which May Exacerbate Seizures ............. 21 Table 5: Clinical Practice Guideline Recommendations for Epilepsy ............. -

Cytochrome P450 Drug Interaction Table
SUBSTRATES 1A2 2B6 2C8 2C9 2C19 2D6 2E1 3A4,5,7 amitriptyline bupropion paclitaxel NSAIDs: Proton Pump Beta Blockers: Anesthetics: Macrolide antibiotics: caffeine cyclophosphamide torsemide diclofenac Inhibitors: carvedilol enflurane clarithromycin clomipramine efavirenz amodiaquine ibuprofen lansoprazole S-metoprolol halothane erythromycin (not clozapine ifosfamide cerivastatin lornoxicam omeprazole propafenone isoflurane 3A5) cyclobenzaprine methadone repaglinide meloxicam pantoprazole timolol methoxyflurane NOT azithromycin estradiol S-naproxen_Nor rabeprazole sevoflurane telithromycin fluvoxamine piroxicam Antidepressants: haloperidol suprofen Anti-epileptics: amitriptyline acetaminophen Anti-arrhythmics: imipramine N-DeMe diazepam Nor clomipramine NAPQI quinidine 3OH (not mexilletine Oral Hypoglycemic phenytoin(O) desipramine aniline2 3A5) naproxen Agents: S-mephenytoin imipramine benzene olanzapine tolbutamide phenobarbitone paroxetine chlorzoxazone Benzodiazepines: ondansetron glipizide ethanol alprazolam phenacetin_ amitriptyline Antipsychotics: N,N-dimethyl diazepam 3OH acetaminophen NAPQI Angiotensin II carisoprodol haloperidol formamide midazolam propranolol Blockers: citalopram perphenazine theophylline triazolam riluzole losartan chloramphenicol risperidone 9OH 8-OH ropivacaine irbesartan clomipramine thioridazine Immune Modulators: tacrine cyclophosphamide zuclopenthixol cyclosporine theophylline Sulfonylureas: hexobarbital tacrolimus (FK506) tizanidine glyburide imipramine N-DeME alprenolol verapamil glibenclamide indomethacin -

Epileptogenesis and Epilepsy
Epileptogenesis and Epilepsy Asla Pitkänen and Xavier Ekolle Ndode-Ekane A.I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland www.tocris.com The word “epilepsy” is derived from the Greek verb ἐπιλαμβάνειν (or epilambánein) meaning “to be seized”, “to be taken hold of”, or “to be attacked”. Hippocrates (400 BC) was the first to suggest that epilepsy is a Products available from Tocris disease of the brain that must be treated. According to the WHO, globally 60 million people have epilepsy, and an estimated 2.4 million are diagnosed with epilepsy each year. There are more than 20 anti-seizure drugs Ca2+-Activated Potassium Channels on market, but in about 30% of people with epilepsy, seizures are not controlled by medication. Apamin, 1-EBIO Ca2+-ATPase Paxilline, Thapsigargin Terminology Molecular, Cellular and Neuronal Network Pathologies CB1 Receptors ACEA, AM 251, (-)-Cannabidiol, Seizure A transient occurrence of signs and/or symptoms due to abnormal excessive Epileptogenesis can be initiated, for example, by an “epilepsy gene”, various types of acute SR141716A or synchronous neuronal activity in the brain. Seizures are categorized according to brain insults or chronic neurodegenerative diseases. The entire epileptogenic process is Cyclooxygenase the International League Against Epilepsy (ILAE) classification into three types: modulated by an individual’s genetic background, microbiota, and exposome (non-genetic Celecoxib, Resveratrol generalized onset; focal onset (previously known as partial seizures); and unknown exposures of an individual in a lifetime, e.g., life-style, medications etc.). Epileptogenesis Gap Channels onset. Epilepsy gene Genetic background continues after epilepsy diagnosis (i.e., occurrence of the first unprovoked seizure) and leads Gap19 to various outcomes (SUDEP, sudden unexpected death; QoL, quality-of-life; Rx, treatment). -
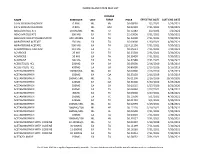
Rhode Island State Mac List Name Strength Unit Dosage
RHODE ISLAND STATE MAC LIST DOSAGE NAME STRENGTH UNIT FORM PRICE EFFECTIVE DATE LAST CHG DATE 0.9 % SODIUM CHLORIDE 0.90% ML HV $0.08050 1/2/2021 1/29/2021 0.9 % SODIUM CHLORIDE 0.90% ML HM $0.02220 7/31/2021 7/30/2021 ABACAVIR SULFATE 20 MG/ML ML SJ $0.52283 1/2/2021 1/1/2021 ABACAVIR SULFATE 300 MG EA TA $1.03096 7/31/2021 7/30/2021 ABACAVIR SULFATE/LAMIVUDINE 600-300MG EA TA $6.23000 7/31/2021 7/30/2021 ABIRATERONE ACETATE 250 MG EA TA $5.00000 1/30/2021 4/30/2021 ABIRATERONE ACETATE 500 MG EA TA $124.11200 7/31/2021 7/30/2021 ACAMPROSATE CALCIUM 333 MG EA TE $0.65613 7/31/2021 7/30/2021 ACARBOSE 25 MG EA TA $0.23718 7/31/2021 7/30/2021 ACARBOSE 50 MG EA TA $0.39450 7/31/2021 7/30/2021 ACARBOSE 100 MG EA TA $0.42948 7/31/2021 7/30/2021 ACEBUTOLOL HCL 200MG EA CA $0.65000 2/16/2019 2/15/2019 ACEBUTOLOL HCL 400MG EA CA $0.86000 2/16/2019 2/15/2019 ACETAMINOPHEN 100MG/ML ML SO $0.07800 2/16/2019 2/15/2019 ACETAMINOPHEN 650MG EA QA $0.35158 2/16/2019 2/15/2019 ACETAMINOPHEN 160MG/5ML ML SE $0.01100 2/16/2019 10/30/2020 ACETAMINOPHEN 120MG EA QA $0.31600 5/30/2020 5/29/2020 ACETAMINOPHEN 500MG EA CA $0.03565 6/27/2020 6/26/2020 ACETAMINOPHEN 650MG EA TS $0.06960 2/27/2021 5/28/2021 ACETAMINOPHEN 80MG EA TC $0.03600 3/27/2021 3/26/2021 ACETAMINOPHEN 160MG EA TC $0.10500 5/1/2021 4/30/2021 ACETAMINOPHEN 325MG EA TA $0.01710 5/1/2021 7/30/2021 ACETAMINOPHEN 160MG/5ML ML SC $0.02093 5/29/2021 5/28/2021 ACETAMINOPHEN 1000MG/100 ML HP $0.12120 5/29/2021 5/28/2021 ACETAMINOPHEN 160MG/5ML ML SL $0.02509 7/31/2021 7/30/2021 ACETAMINOPHEN 500MG EA TA $0.03020 7/31/2021 7/30/2021 ACETAMINOPHEN 1000MG/100 ML HV $0.35527 7/31/2021 7/30/2021 ACETAMINOPHEN WITH CODEINE 12-120MG/5 ML SJ $0.01807 5/19/2018 5/31/2019 ACETAMINOPHEN WITH CODEINE 30-300MG EA TA $0.11199 7/31/2021 7/30/2021 ACETAMINOPHEN WITH CODEINE 15-300MG EA TA $0.16165 7/31/2021 7/30/2021 ACETAMINOPHEN WITH CODEINE 60-300MG EA TA $0.20461 7/31/2021 7/30/2021 ACETAZOLAMIDE 250MG EA TA $0.54052 7/31/2021 7/30/2021 The RI State MAC list is updated approximately monthly.