An Increase in the Levels of Middle Surface Antigen Characterizes Patients Developing HBV-Driven Liver Cancer Despite Prolonged Virological Suppression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rankings Municipality of Gaeta
9/24/2021 Maps, analysis and statistics about the resident population Demographic balance, population and familiy trends, age classes and average age, civil status and foreigners Skip Navigation Links ITALIA / Lazio / Province of Latina / Gaeta Powered by Page 1 L'azienda Contatti Login Urbistat on Linkedin Adminstat logo DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA Municipalities Powered by Page 2 Aprilia Stroll up beside >> L'azienda Contatti Login Urbistat on Linkedin Lenola AdminstatBassiano logo DEMOGRAPHY ECONOMY RANKINGS SEARCH Maenza Campodimele ITALIA Minturno Castelforte Monte San Cisterna di Biagio Latina Norma Cori Pontinia Fondi Ponza Formia Priverno Gaeta Prossedi Itri Rocca Massima Latina Roccagorga Roccasecca dei Volsci Sabaudia San Felice Circeo Santi Cosma e Damiano Sermoneta Sezze Sonnino Sperlonga Spigno Saturnia Terracina Ventotene Provinces FROSINONE RIETI LATINA ROMA VITERBO Regions Powered by Page 3 Abruzzo Liguria L'azienda Contatti Login Urbistat on Linkedin AdminstatBasilicata logo Lombardia DEMOGRAPHY ECONOMY RANKINGS SEARCH Calabria MarcheITALIA Campania Molise Città del Piemonte Vaticano Puglia Emilia-Romagna Repubblica di Friuli-Venezia San Marino Giulia Sardegna Lazio Sicilia Toscana Trentino-Alto Adige/Südtirol Umbria Valle d'Aosta/Vallée d'Aoste Veneto Municipality of Gaeta Territorial extension of Municipality of GAETA and related population density, population per gender and number of households, average age and incidence of foreigners TERRITORY DEMOGRAPHIC DATA (YEAR 2019) Region Lazio Province Latina Inhabitants (N.) 20,071 Sign Province LT Families (N.) 9,217 Hamlet of the Males (%) 48.0 0 municipality Females (%) 52.0 Surface (Km2) 29.20 Foreigners (%) 4.4 Population density 687.3 Average age (Inhabitants/Kmq) 47.9 (years) Powered by Page 4 Average annual L'azienda Contatti Login Urbistat on Linkedin variation -0.84 Adminstat logo (2014/2019) DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA MALES, FEMALES AND DEMOGRAPHIC BALANCE FOREIGNERS INCIDENCE (YEAR 2019) (YEAR 2019) Balance of nature [1], Migrat. -

Urban Society and Communal Independence in Twelfth-Century Southern Italy
Urban society and communal independence in Twelfth-Century Southern Italy Paul Oldfield Submitted in accordance with the requirements for the degree of PhD. The University of Leeds The School of History September 2006 The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. Acknowledgements I would like to express my thanks for the help of so many different people, without which there would simply have been no thesis. The funding of the AHRC (formerly AHRB) and the support of the School of History at the University of Leeds made this research possible in the first place. I am grateful too for the general support, and advice on reading and sources, provided by Dr. A. J. Metcalfe, Dr. P. Skinner, Professor E. Van Houts, and Donald Matthew. Thanks also to Professor J-M. Martin, of the Ecole Francoise de Rome, for his continual eagerness to offer guidance and to discuss the subject. A particularly large thanks to Mr. I. S. Moxon, of the School of History at the University of Leeds, for innumerable afternoons spent pouring over troublesome Latin, for reading drafts, and for just chatting! Last but not least, I am hugely indebted to the support, understanding and endless efforts of my supervisor Professor G. A. Loud. His knowledge and energy for the subject has been infectious, and his generosity in offering me numerous personal translations of key narrative and documentary sources (many of which are used within) allowed this research to take shape and will never be forgotten. -
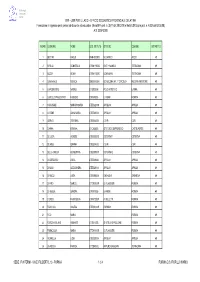
MPI - USR PER IL LAZIO - UFFICIO SCOLASTICO PROVINCIALE DI LATINA Formazione in Ingresso Per Il Personale Docente Ed Educativo (Nota MPI Prot
Gilda degli insegnanti Latina MPI - USR PER IL LAZIO - UFFICIO SCOLASTICO PROVINCIALE DI LATINA Formazione in ingresso per il personale docente ed educativo (Nota MPI prot. n. 3577 del 28.02.08 e Nota USR Lazio prot. n. 4302 del 03.03.08) A.S. 2007-2008 N ORD COGNOME NOME COD. ISTITUTO ISTITUTO COMUNE DISTRETTO 1 BETTINI PAOLO RMRH030003 M.G.APICIO ANZIO 49 2 BIFOLCI DONATELLA LTMM11900Q SINTI + MAMELI TERRACINA 49 3 BOZZA SONIA LTMM11300R DON MILANI TERRACINA 49 4 CANNAVALE MONICA SAEE08400N DD NOCERA INF. 1° CIRCOLO NOCERA INFERIORE 49 5 CAPODIFERRO NATALE LTIS00900B POLO ARTISTICO LATINA 49 6 CARDILLO PIACENTINO FABRIZIO LTRI010006 E. FERMI FORMIA 49 7 CARDINALE MARIAROSARIA LTEE02200Q APRILIA 3 APRILIA 49 8 CATONE ANNA MARIA LTEE020004 APRILIA 1 APRILIA 49 9 CIERVO STEFANIA LTEE034002 C O R I CORI 49 10 D'ANNA ERMINIA LTIC825005 ISTITUTO COMPRENSIVO CASTELFORTE 49 11 DE LUCA AGNESE LTEE02900E CISTERNA 1 CISTERNA 49 12 DE MEO GIANNA LTEE034002 C O R I CORI 49 13 DELLA CHIESA GIUSEPPINA LTEE03000P CISTERNA 2 CISTERNA 49 14 DI GREGORIO LUCIA LTEE020004 APRILIA 1 APRILIA 49 15 DI MAIO ALESSANDRA LTEE020004 APRILIA 1 APRILIA 49 16 DI NUCCI LUISA LTEE09000A SABAUDIA SABAUDIA 49 17 DI PIRO MARCO LTTD01000R G. FILANGIERI FORMIA 49 18 DI VEGLIA SANDRA LTRI010006 E. FERMI FORMIA 49 19 D'URSO FRANCESCA LTRH01000P A. CELLETTI FORMIA 49 20 FAVOCCIA ANGELA LTEE04300R FORMIA 1 FORMIA 49 21 FICO MARIA FORMIA 49 22 FOROGHI BILAND KIUMARS LTIS01100B IS VITRUVIO POLLIONE FORMIA 49 23 FRANCIOSA MARIA LTTD01000R G. FILANGIERI FORMIA 49 24 GONNELLA LIDIA LTEE020004 APRILIA 1 APRILIA 49 25 LA ROCCA FRANCA LTTD04000L ARTURO BIANCHINI TERRACINA 49 SEDE: IPIA FERMI - VIA E. -

Le Golfe Gaeta
AZIENDA DI PROMOZIONE TURISTICA DELLA PROVINCIA DI LATINA SOMMARIO IL GOLFO DI GAETA Informazioni p. 1 Come arrivare p. 2 Il Golfo di Gaeta p. 4 Le località p. 8 Le Isole Pontine p. 22 Sport e tempo libero p. 24 Stabilimenti balneari p. 26 Locali notturni e ritrovi tipici p. 27 Folklore e manifestazioni p. 28 Gastronomia p. 30 Ambiente e natura p. 32 Per informazioni sull’area e sulle località contenute nella pubblicazione, rivolgersi ai seguenti uffici turistici: Pour tous reinsegnements sur la zone et sur les localités présentées dans cette publication, s’adresser aux bureaux de tourisme suivantes: A.P.T. – Azienda di Promozione Turistica della Provincia di Latina Via Legnano, 42 04100 LATINA – Tel. 0773695404 – Fax 0773661266 www.aptlatinaturismo.it e www.latinaturismo.it - e-mail: [email protected] Ufficio Informazioni: Piazza del Popolo – Tel. 0773480672 I.A.T. – Uffici Informazioni e Assistenza al Turista - Bureaux de renseignements Ideazione Formia Pier Giacomo Sottoriva Viale Unità d’Italia, 30 – 34 (C.A.P. 04023) Tel. 0771771490 - Fax 0771323275 Testi Gaeta Maria Caterina Santini Via E. Filiberto, 5 (C.A.P. 04024) – Tel. 0771461165 – Fax 0771450779 Foto Minturno–Scauri Archivio APT Latina, P. Petrignani, Via Lungomare, 32 (C.A.P. 04028) – Tel. 0771683788 – Fax 0771620829 P. Silvano, L. Orilia, Terracina S. Vannini, Archivio IGDA Via Leopardi (C.A.P. 04019) – Tel. 0773727759 – Fax 0773721173 Coordinamento redazionale Bruno Maragoni, Anna Maria ORGANISMI TURISTICI DI CATEGORIA/ORGANISMES TOURISTIQUES DE CATÉGORIE Mantione Associazione Provinciale Albergatori – c/o Confcommercio Grafica Via dei Volsini, 46 – 04100 Latina – Tel. 0773610678 Alberto Costa, Marco Stingo Traduzioni Confcommercio Latina – Via dei Volsini, 46 – 04100 Latina – Tel. -

A Cosmatesque Architectural Fragment Inlaid with a Trelliswork Design Italy
A Cosmatesque architectural fragment inlaid with a trelliswork design Italy, Gaeta, Cathedral of Sant'Erasmo and San Marciano First quarter 13th century, reusing ancient stone 67.5 x 20 x 6.5 cm; a single panel of delicately veined grey-white stone consistent with Proconnesian marble, inset with red porphyry (perfido rosso), green porphyry (marmor lecdaemon), rosso antico, and cut glass tesserae. Provenance Probably made as part of a liturgical screen for the cathedral of Sant'Erasmo and San Marciano, Gaeta, Lazio; Likely removed from the cathedral and reinstalled at the church of Santa Lucia in or shortly after 1648, when the cathedral’s interior was renovated; Private collection, Italy; Collection of Ogden Smith, London, by c. 1960 This slender architectural panel is decorated with a wide band of purplish red porphyry, green serpentine, and turquoise tesserae inlaid in a complex pattern of stars and trellis work. The design turns a sharp corner near the top of the slab and disappears off of its left-hand edge above a series of leaf and tongue mouldings carved in low relief. The combination of these features tells us we are looking at a fragment of a much larger object, and yet its neatly finished edges also show that whatever it comes from was carefully constructed from a series of blocks that must have slotted into place alongside one another. Cosmati work, ‘Sectilia’ ornament, and ancient stone – use and reuse in the Medieval world The panel’s inlaid decoration conforms to a style of ornament often described as Cosmati work, a name given to inlaid geometric ‘sectilia’ stonework typical of the architecture of Early Medieval Italy, and especially of Rome and its surroundings (figs. -

Women in Court in Medieval Southern Italy'
Disputes and Disparity: Women in Court in Medieval Southern Italy' Patricia Skinner University of Southampton The backbone of many localized medieval studies is the evidence furnished by charters - records of land transactions - surviving from the area under scrutiny. The survival of charter evidence is itself patchy: much more material survives from Italy than from France or England up to 1300, for example, and the copious archives of Catalonia and north-western Spain are also providing a rich seam of information for medieval historians to mine. Charter collections frequently include records of court cases before local officials, and these can be extremely illuminating when examining the lives and status of women in a medieval community. Of particular value is their evidence for women's voices, protesting against a given situation. This paper will examine several important questions. Firstly, what access did women have to courts and under what circumstances might they bring a case? When they reached the court, did their tactics show any variation from men's? And fmally, how successful were women in winning their cases when compared with men at court? In addition, I shall consider whether court case records are a reliable source of evidence for women's lives in general, or if a woman in court was in fact an exceptional occurrence. In southern Italy, the survival of much documentation from the ninth to twelfth centuries allows us to examine these issues in some detail, but the area needs to be set in a wider European context. The problems raised here are methodological as well as empirical. -

Federazione Italiana Pallacanestro Comitato Provinciale Latina
FEDERAZIONE ITALIANA PALLACANESTRO COMITATO PROVINCIALE LATINA COMUNICATO UFFICIALE N. 12 DEL 16 OTTOBRE 2009 UFFICIO GARE N. 980003 Campionato Regionale Under 17 Maschile Calendario Ufficiale Gare Girone: GIRONE A Gara n. TURNO DI ANDATA 1 Giornata 195 SABOTINO BASKET - BASKET SERAPO 85 Lun 02-11-2009 20:30 SCUOLA. VITO FABIANO - Via Don Vincenzo Onorati Snc - BORGO SA 196 IL COCCODRILLO FORMIA - AB LATINA Dom 01-11-2009 16:30 PALESTRA - S.rocco - Trav. Cinole - MARANOLA DI FORMIA (LT) 197 I GIGANTI DEL MB - GIOCOBASKET SABAUDIA Sab 31-10-2009 17:00 PALAZZETTO DELLO SPORT - Viale Europa - TERRACINA (LT) 198 A.S.D. VIRTUS CISTERNA - A.S.D. BASKET TERRACINA Mer 28-10-2009 19:15 PALESTRA S.M. PLINIO - Via Dei Bersaglieri - CISTERNA DI LATINA (L 2 Giornata 199 AB LATINA - I GIGANTI DEL MB Dom 08-11-2009 11:30 PALACECI - Via dei Mille - LATINA (LT) 200 A.S.D. BASKET TERRACINA - SABOTINO BASKET Dom 08-11-2009 16:00 PALAZZETTO DELLO SPORT - Viale Europa - TERRACINA (LT) 201 BASKET SERAPO 85 - A.S.D. VIRTUS CISTERNA Dom 08-11-2009 15:30 PALAMARINA - Via Marina Di Serapo - GAETA (LT) 202 GIOCOBASKET SABAUDIA - IL COCCODRILLO FORMIA Sab 07-11-2009 16:30 PALL. TENSOSTATICO - Via Cesare Del Piano - SABAUDIA (LT) 3 Giornata 203 A.S.D. BASKET TERRACINA - BASKET SERAPO 85 Dom 15-11-2009 16:00 PALAZZETTO DELLO SPORT - Viale Europa - TERRACINA (LT) 204 SABOTINO BASKET - AB LATINA Lun 16-11-2009 20:30 SCUOLA. VITO FABIANO - Via Don Vincenzo Onorati Snc - BORGO SA 205 IL COCCODRILLO FORMIA - I GIGANTI DEL MB Dom 15-11-2009 16:30 PALESTRA - S.rocco - Trav. -

Fondi E Sperlonga): Punti Presa Caccia Al Cinghiale in Braccata
DISTRETTO 2 (comprendente i comuni di Formia, Gaeta, Itri, Fondi e Sperlonga): Punti Presa caccia al cinghiale in braccata : Punto presa n.6 composto da 3 sottozone “ Carrrubo” di Ha 19 2 , “ Campese “ di Ha 10 1 e “Vate Cancello” di Ha 2 79 - (Formia); Punto presa n.7 composto da 2 sottozone “ Sugh erete” di Ha 2 79 - (Formia) e “ Fossato Aratro – ex Moneta” di Ha 6 6 - (Itri); Punto presa n. 8 “S. Onofrio/ Schettino” di Ha 4 65 - (Itri); Punto presa n. 9 “Marano ” di Ha 5 32 - (Itri); Punto presa n.10 composto da 2 sottozone “Pozzel le” di Ha 403 (Sperlonga/ Itri) e “Pietra Grossa” di Ha 113 - (Fondi) ; Punto presa n.11 “Vallaneto” di Ha 472 - (Fondi) ; Punto presa n.12 A “Rene” di Ha 265 - (Fondi) ; Punto presa n.12 B “Vallevigna” di Ha 188 - (Fondi) Punti Presa caccia al cinghiale in girata: Zona “1 ” composta da 5 so ttozone “Polveriera” di Ha 36 ricadente nel comprensorio di “girata” del Comune di Gaeta , “Cuccurone” di Ha 12, “Costaforte nord” di Ha 17, “Costaforte sud” di Ha 20 ricadente nel comprensorio di “girata” del Comune di Itri ; Aree di caccia al cinghiale in selezione : a) S1 “Caserma CC” di Ha 6 a Itri b) S2 “Stazione” di Ha 6 a Itri c) S3 “Valle scura” di Ha 5 7 a Itri d) S4 “Monte Rotondo” di Ha 1 3 a Fondi e) S5 “Monte Valletonda” di Ha 10 a Fondi f) S6 “Santa Porpora” di Ha 1 4 a Fondi SISTEMA INFORMATIVO ATC LATINA 2 Legenda IBA RAMSAR EUAP (PARCHI) ZPS SIC-ZSC CONFINI ZRV Zone Caccia Selezione Zona Girata Parco Naturale Regionale Incendi 2009 Incendi 2010 Incendi 2011 Incendi 2012 Incendi 2013 Incendi 2014 Incendi -

Ecco Gli Esercenti Di Gaeta Che, Ad Ora, Hanno Aderito All'iniziativa: SUPERMERCATI/ALIMENTARI/ENOTECHE 1. Mister Risparmio
Ecco gli esercenti di Gaeta che, ad ora, hanno aderito all'iniziativa: SUPERMERCATI/ALIMENTARI/ENOTECHE 1. Mister risparmio Via Begani 2 Gaeta 0771.465102 2. Supermercato Natale di Massimo Natale Corso Italia - Gaeta 0771.902007 3. Ninotto Via Genova, n.4 - Gaeta 0771.460901 4. Lo Spicchio di Anna Capobianco Via Indipendenza 8 - Gaeta 0771.463616/327.9829506 5. Caseificio "Lo spicchio - La Pupatella Srl" Lungomare Caboto, n. 316 0771.471026 Via Papa Giovanni XXIII 0771.465360 6. Alimentari il Golosone di Strozza Loreta Via indipendenza 50 - Gaeta 0771.464477 7. Enoteca Ciccariello Via Sant'Agostino 41, - Gaeta 0771.470095/329.7229604 8. Enoteca Perrone Corso Cavour - Gaeta 338.7569792 9. Panificio Calegna Via Calegna, n. 1 - Gaeta 0771.712107 10. Ottimo Market medievale Piazza Traniello, n. 20 - Gaeta 0771.464722 11. Punto Casa Via Calegna, n.7 0771.712090/333.1061842 12. Uno risparmi sicuro Corso Italia, n. 71 - Gaeta 0771.741230 13. Uno risparmio sicuro Lungomare Caboto, n. 440 - Gaeta 0771.466380 14. Alimentari "Mary" via Buonomo, n. 49 - Gaeta 347.6546240/327.9558791 15. "Risparmione" Via Indipendenza, n. 126 333.9021536 FARMACIA 16. Farmacia "Gualtieri" Lungomare Caboto, n. 556 - Gaeta 0771.460543 FRUTTERIE 17. Frutteria Vaudo Veronica Via Indipendenza 45 - Gaeta 0771.209609 18. Frutta e verdura da Antonio Via Papa Giovanni XXIII n°3 Gaeta 333 4723351 19. Frutteria San Nilo di Paliotta Roberto Via Rimini 1 - Gaeta 380.3407757 20. L'Isola della frutta Via Calegna - Gaeta 349.2684551 21. Lungomare della frutta Lungomare Caboto, snc 324.5881689 GIOCHERIE 22. ToyStore giocattoli Via Faustina, n. -

C.S. Coronavirus 14 Ottobre N
DIREZIONE GENERALE COMUNICATO STAMPA Rispetto alla giornata di ieri, si registrano 47 nuovi casi positivi , distribuiti nei Comuni di Aprilia ( 21), di Cisterna di Latina (2), di Cori (3), di Fondi ( 1), di Gaeta (4) di Latina (4), di Minturno ( 1), di Pontinia (1), di Sermoneta (3), di Sperlonga (1), di Sonnino (1) e di Terracina (5). Non si registrano nuovi decessi. Casi Prevalenza Guariti Deceduti Positivi di cui a domicilio 1769 30,75 697 41 1031 926 Si ricorda che, in relazione alla situazione epidemiologica in essere localmente, sono stati attivati nei Comuni di Aprilia, Gaeta e Priverno, locali postazioni permanenti di "Drive in" per l’esecuzione del test antigenico o molecolare volto alla rilevazione del coronavirus SARS -COV2. GAETA: attivo dalle ore 9:00 alle ore 15:00 dal lunedì al sabato c/o ex Ospedale Monsignor Don Luigi di Liegro via salita cappuccini APRILIA : attivo dalle ore 9:00 alle ore 14:00 dal lunedì al sabato c/o Fiera (mercato dei fiori) località Campoverde Km 46,600 (direzione Latina) della SS.148 Pontina PRIVERNO: attivo dalle ore 8:30 alle ore 13:30 dal lunedì al sabato c/o la Casa della Salute sita in via Madonna delle Grazie L'accesso al “drive in” è regolato da prenotazione che dovrà essere effettuata almeno 24 h prima. Pertanto non sarà garantito il tampone alle persone prive di prenotazione, ad eccezione dei seguenti cittadini che potranno presentarsi senza prenotazione: - Cittadini che devono partire per Stati Esteri, dove viene richiesta idonea certificazione, per i quali sarà sufficiente mostrare al personale del “drive in” idoneo titolo di viaggio e ricevuta di pagamento; - Cittadini contattati dal Dipartimento di Prevenzione, che procederà direttamente ad effettuare la prenotazione. -

Senato Della Repubblica X Legislatura
SENATO DELLA REPUBBLICA X LEGISLATURA N. 1767 DISEGNO DI LEGGE d'iniziativa dei senatori BERNARDI, VENTRE, SARTORI, CAPPELLI e TANI COMUNICATO ALLA PRESIDENZA IL 19 MAGGIO 1989 Istituzione del tribunale di Gaeta ONOREVOLISENATORI.~ Il presente disegno di che, allo stato, è servita, a differenza di altre legge trova le sue premesse nella unanime zone del Lazio, di analoga densità abitativa aspirazione delle cittadinanze di quattordici (basti citare i tribunali di Civitavecchia e comuni della parte meridionale ed insulare Velletri in provincia di Roma e quello di della provincia di Latina ad usufruire, ai fini di Cassino nel Sud frusinate), da un solo tribuna~ una più sollecita, efficiente ed accessibile le, avente sede nel capoluogo e quindi ubicato giustizia, di una sede di tribunale agevolmente quasi all'estremità settentrionale del vasto cir~ raggiungibile. condario. L'istanza proviene dalle popolazioni, dai Le distanze tra Latina e le tre sedi di pretura consigli comunali e dagli operatori giuridici del «Sud pontino», Fondi, Gaeta e Minturno, del cosiddetto «Sud pontino», quella fascia sono rispettivamente di 60, 73 e 93 chilometri; del litorale tirrenico e dell'immediato entro~ taluni centri abitati, sulla riva destra del terra, in passato facente parte del territorio Garigliano (Castelforte, Santi Cosma e Damia~ campano, compresa tra la piana di Fondi ed il no e relative frazioni) distano circa 110 fiume Garigliano,' estesa 66.749 ettari e popo~ chilometri dal suddetto capoluogo, e parados~ lata da 134.856 abitanti (dati del censimento salmente, sono molto più vicini ad altre sedi di dell'ottobre 1981), su complessivi 225.043 tribunale (Cassino a circa 30 chilometri, Santa ettari e 434.086 abitanti dell'intera provincia, Maria Capua Vetere a 60, Frosinone a 80). -

Liberty Bitcoin Youth Foiling World Cup
Liberty Bitcoin Youth Foiling World Cup Gaeta - Italy 24th February – 12th March 2021 Liberty Bitcoin Youth Foiling World Cup 69F Team, Yacht Club Gaeta, Italian Sailing Federation (FIV), Royal Hong Kong Yacht Club (RHKYC), proudly invites your Yacht Club and your Country to participate in the inaugural event of the Liberty Bitcoin Youth Foiling World Cup in Gaeta - Italy from 24 February to 12 March 2021. We are inviting mixed/female teams of 3 or 4 people, age 18-25 from across the world to participate. With the latest generation fast foiling monohull boats, the Persico 69F, an innovative race format, jury on the water, professional content production and social media sharing, we believe key ingredients are in place to create a globally leading arena for World's best Youth Foiling sailors. The event will take place over almost three weeks. The first week will feature training sessions. The two following weeks will feature 8 days of racing. The top 6 teams will qualify for the finals. The finals will consist of fleet races, scheduled to be completed over two days. The team entry cost is 10.500 Euro which includes all racing over the period and training sessions onboard the Persico 69F ahead of the events. With strong support from the 69F shore team all boats will continuously be maintained and ready to sail. We expect most of the teams to arrive early February in Gaeta. With so many sailing events being cancelled, including the Youth America's Cup, we aim to make this a unique event where youth sailors from all over the world come together, to share and learn from each other and develop long lasting friendships.