Effects of Maturation and Processing Technologies on Nutritional and Sensory Qualities of Itrana Table Olives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rankings Municipality of Gaeta
9/24/2021 Maps, analysis and statistics about the resident population Demographic balance, population and familiy trends, age classes and average age, civil status and foreigners Skip Navigation Links ITALIA / Lazio / Province of Latina / Gaeta Powered by Page 1 L'azienda Contatti Login Urbistat on Linkedin Adminstat logo DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA Municipalities Powered by Page 2 Aprilia Stroll up beside >> L'azienda Contatti Login Urbistat on Linkedin Lenola AdminstatBassiano logo DEMOGRAPHY ECONOMY RANKINGS SEARCH Maenza Campodimele ITALIA Minturno Castelforte Monte San Cisterna di Biagio Latina Norma Cori Pontinia Fondi Ponza Formia Priverno Gaeta Prossedi Itri Rocca Massima Latina Roccagorga Roccasecca dei Volsci Sabaudia San Felice Circeo Santi Cosma e Damiano Sermoneta Sezze Sonnino Sperlonga Spigno Saturnia Terracina Ventotene Provinces FROSINONE RIETI LATINA ROMA VITERBO Regions Powered by Page 3 Abruzzo Liguria L'azienda Contatti Login Urbistat on Linkedin AdminstatBasilicata logo Lombardia DEMOGRAPHY ECONOMY RANKINGS SEARCH Calabria MarcheITALIA Campania Molise Città del Piemonte Vaticano Puglia Emilia-Romagna Repubblica di Friuli-Venezia San Marino Giulia Sardegna Lazio Sicilia Toscana Trentino-Alto Adige/Südtirol Umbria Valle d'Aosta/Vallée d'Aoste Veneto Municipality of Gaeta Territorial extension of Municipality of GAETA and related population density, population per gender and number of households, average age and incidence of foreigners TERRITORY DEMOGRAPHIC DATA (YEAR 2019) Region Lazio Province Latina Inhabitants (N.) 20,071 Sign Province LT Families (N.) 9,217 Hamlet of the Males (%) 48.0 0 municipality Females (%) 52.0 Surface (Km2) 29.20 Foreigners (%) 4.4 Population density 687.3 Average age (Inhabitants/Kmq) 47.9 (years) Powered by Page 4 Average annual L'azienda Contatti Login Urbistat on Linkedin variation -0.84 Adminstat logo (2014/2019) DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA MALES, FEMALES AND DEMOGRAPHIC BALANCE FOREIGNERS INCIDENCE (YEAR 2019) (YEAR 2019) Balance of nature [1], Migrat. -

DGR Lazio Riclassificazione Sismica N.766 01/08/03
REGIONE LAZIO GIUNTA REGIONALE DELIBERAZIONE N. 766 DEL 01.08.2003 Dipartimento Territorio STRUTTURA Direzione Regionale Ambiente e Protezione Civile PROPONENTE Area 08 - Difesa del Suolo e Servizio Geologico Regionale Prot. n. _______________ del _________________ OGGETTO: Riclassificazione sismica del territorio della Regione Lazio in applicazione dell’Ordinanza del Presidente del Consiglio dei Ministri n° 3274 del 20 marzo 2003. Prime disposizioni Geol. A. Colombi Geol. F. Meloni Geol. F. Nolasco Geol. F. Nolasco Dr. R. De Filippis Ing. P. Cuccioletta Prof. V. Saraceni Geol. A. Orazi L’ESTENSORE IL RESP. PROCEDIMENTO IL DIRIGENTE DI AREA IL DIRETTORE REG.LE IL DIRETTORE DI DIPARTIMENTO L’ASSESSORE Dipartimento: DI CONCERTO ______________________________________________________________ __________________ _____________________ IL DIRETTORE DI DIPARTIMENTO L’ASSESSORE ALL’ESAME PREVENTIVO COMM.NE CONS.RE ALL’ESAME PREVENTIVO COLL. REVISORI COMMISSIONE CONSILIARE: COLLEGIO REVISORI: Data dell’esame: _________________ Data dell’esame: ___________________ con osservazioni senza osservazioni con osservazioni senza osservazioni SEGRETERIA DELLA GIUNTA Data di ricezione _______________________ ISTRUTTORIA: ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ _________________________________ ___________________________ IL RESPONSABILE DEL PROCEDIMENTO IL DIRIGENTE COMPETENTE ______________________________ -

Oscar Soc. Coop. Agricola Loc
Oscar Soc. Coop. Agricola Loc. Basso Le Case, 2 – 04010 Rocca Massima (LT) Tel/Ph. +39. 06.9664883 – 339.6627231 email: [email protected] Web: http://www.oscarfrantoio.com Our company Azienda Oscar is a young, family-run olive farm overlooking the valley of Rocca Massima, a small ancient village in the heart of the Lepini Mountains in the province of Latina. Located at an elevation of 450 meters above sea level, it is set in olive groves in one of Latium’s most scenic inland areas, a stone’s throw from Rome. This is Latium’s ancient heartland, the home of the Itrana olive, one of our local treasures. This is the setting in which our family has grown table olives and made olive oil for generations. The farm boasts process and service certifications such as UNI EN ISO 22005:2008. Our products “Carventum” Colline Pontine PDO extravirgin olive oil available in 0.50 litre bottles. Salt-cured Gaeta olives (ingredients: White Itrana olives, water, salt); Salt.-cured white Itrana table olives (ingredients: White Itrana olives, water, salt); White Itrana olive paté (ingredients: White Itrana olives, extravirgin olive oil, salt) available in 180 g and 500 g jars Gaeta olive paté (ingredients: Itrana olives, extravirgin olive oil, salt) available in 180 g and 500 g jars Our products are available all year round. They are sold at our sales point and distributed locally, and are also available at sales outlet elsewhere in Latium and Italy. Our products are exported to non-EU countries, and we can arrange shipping to Italy and abroad. -

Nome Indirizzo CONTRADA MUSTAGA, 19 – 04020 ITRI (LT
CURRICULUM VITAE INFORMAZIONI PERSONALI Nome ROBERTO DI PERNA Indirizzo CONTRADA MUSTAGA , 19 – 04020 I TRI (LT) - V IA ITALO MONTEMEZZI ,3 00164 R OMA (RM) Telefono 06-50932458 cellulare 347-4687857 abitazione 0771-721534 Fax 06-50933658 E-mail [email protected] ; [email protected] Nazionalità Italiana Luogo e Data di nascita FORMIA 27, giugno 1967 Codice Fiscale DPRRRT67H27D708P ESPERIENZA LAVORATIVA • Date (da – a) DAL 2005 AL 2013 • Tipo di azienda o settore Società (srl,sas,snc), cooperative, consorzi per opere di urbanizzazione, associazioni onlus, ditte individuali, lavoratori autonomi, professionisti. • Tipo di impiego Libero professionista Consulente amministrativo-contabile-fiscale • Principali mansioni e responsabilità Collaborazione con studi di consulenza aziendale e fiscale: predisposizione di: dichiarazione dei redditi e IVA – Bilanci – Contabilità ordinaria, semplificata, forfettaria, – Contabilità pubblica; redazione di business plan, predisposizioni progetti di finanziamento Regione Lazio –Sviluppo Italia–Filas-SG Lazio, rendicontazione progetti europei e regionali. • Date (da – a) DAL 2000 AL 2013 • Tipo di azienda o settore Comune di Ventotene e Riserva Naturale Statale e Area Marina Protetta del Comune di Ventotene. Ente pubblico territoriale e Parco. • Tipo di impiego Consulente amministrativo-contabile-finanziario • Principali mansioni e responsabilità Collaborazione pluriennale con i responsabili di area degli Enti citati. Responsabile di: predisposizione dichiarazione dei redditi e IVA con gestione separata -IRAP- Bilancio Preventivo, Consuntivo Pluriennale - Relazione Previsionale e Programmatica- adempimenti fiscali e contributivi, tenuta rapporti Co.Co.Co.; adempimenti per la Tesoreria; adempimenti fiscali in relazione alla contribuzione degli Enti verso i dipendenti; adempimenti connessi all’elaborazione delle buste paga dei dipendenti, attività di trasmissione dati via telematica per i servizi finanziari; risoluzione di problematiche connesse all’approvvigionamento delle risorse. -

Urban Society and Communal Independence in Twelfth-Century Southern Italy
Urban society and communal independence in Twelfth-Century Southern Italy Paul Oldfield Submitted in accordance with the requirements for the degree of PhD. The University of Leeds The School of History September 2006 The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. Acknowledgements I would like to express my thanks for the help of so many different people, without which there would simply have been no thesis. The funding of the AHRC (formerly AHRB) and the support of the School of History at the University of Leeds made this research possible in the first place. I am grateful too for the general support, and advice on reading and sources, provided by Dr. A. J. Metcalfe, Dr. P. Skinner, Professor E. Van Houts, and Donald Matthew. Thanks also to Professor J-M. Martin, of the Ecole Francoise de Rome, for his continual eagerness to offer guidance and to discuss the subject. A particularly large thanks to Mr. I. S. Moxon, of the School of History at the University of Leeds, for innumerable afternoons spent pouring over troublesome Latin, for reading drafts, and for just chatting! Last but not least, I am hugely indebted to the support, understanding and endless efforts of my supervisor Professor G. A. Loud. His knowledge and energy for the subject has been infectious, and his generosity in offering me numerous personal translations of key narrative and documentary sources (many of which are used within) allowed this research to take shape and will never be forgotten. -

Territorio E Popolazione La Provincia Di Latina Si Estende Per 2.250 Kmq, È Costituita Da 33 Comuni E Una Popolazione Residen
Territorio e popolazione La provincia di Latina si estende per 2.250 kmq, è costituita da 33 comuni e una popolazione residente di 569.664 abitanti (Maschi 280.314 e Femmine 289.350), di cui 42.821 stranieri (Fonte Dati ISTAT al 31 dicembre 2013) Il territorio, diviso tra aree collinari, montuose e piane costiere comprende anche le isole dell’arcipelago pontino. Il 67% della popolazione risiede in pianura, il 32% in collina e l’1% circa in montagna e nelle isole dell’arcipelago pontino. La densità abitativa della provincia di Latina è di 245 abitanti/kmq con una variabilità di 722 abitanti/kmq nel comune di Gaeta e di 17 abitanti/kmq nel comune di Campodimele. La provincia di Latina si caratterizza per una popolazione giovane con un’età media di 42,7 anni e un indice di vecchiaia (i.v.) pari a 133 il più basso del Lazio. Seppur più lento rispetto alle altre province laziali, è comunque emergente il progressivo invecchiamento della popolazione dovuto alla diminuzione del tasso di natalità e al contemporaneo aumento della sopravvivenza e speranza di vita. Dall’analisi degli indicatori di struttura della popolazione per zone altimetriche emerge che le classi d’età più giovani dai 15 ai 64 anni prevalgono in pianura e in collina. In montagna e nelle isole prevale la popolazione di età superiore ai 65 anni.Il territorio dell’Azienda Unità Sanitaria Locale di Latina è organizzato in 5 Distretti Sanitari. Il Distretto 1 si caratterizza per un territorio prevalentemente pianeggiante, una popolazione giovane e un’ elevata presenza di stranieri, che nel comune di Aprilia raggiunge l’11,2% della popolazione. -
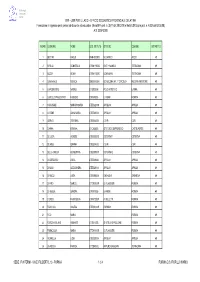
MPI - USR PER IL LAZIO - UFFICIO SCOLASTICO PROVINCIALE DI LATINA Formazione in Ingresso Per Il Personale Docente Ed Educativo (Nota MPI Prot
Gilda degli insegnanti Latina MPI - USR PER IL LAZIO - UFFICIO SCOLASTICO PROVINCIALE DI LATINA Formazione in ingresso per il personale docente ed educativo (Nota MPI prot. n. 3577 del 28.02.08 e Nota USR Lazio prot. n. 4302 del 03.03.08) A.S. 2007-2008 N ORD COGNOME NOME COD. ISTITUTO ISTITUTO COMUNE DISTRETTO 1 BETTINI PAOLO RMRH030003 M.G.APICIO ANZIO 49 2 BIFOLCI DONATELLA LTMM11900Q SINTI + MAMELI TERRACINA 49 3 BOZZA SONIA LTMM11300R DON MILANI TERRACINA 49 4 CANNAVALE MONICA SAEE08400N DD NOCERA INF. 1° CIRCOLO NOCERA INFERIORE 49 5 CAPODIFERRO NATALE LTIS00900B POLO ARTISTICO LATINA 49 6 CARDILLO PIACENTINO FABRIZIO LTRI010006 E. FERMI FORMIA 49 7 CARDINALE MARIAROSARIA LTEE02200Q APRILIA 3 APRILIA 49 8 CATONE ANNA MARIA LTEE020004 APRILIA 1 APRILIA 49 9 CIERVO STEFANIA LTEE034002 C O R I CORI 49 10 D'ANNA ERMINIA LTIC825005 ISTITUTO COMPRENSIVO CASTELFORTE 49 11 DE LUCA AGNESE LTEE02900E CISTERNA 1 CISTERNA 49 12 DE MEO GIANNA LTEE034002 C O R I CORI 49 13 DELLA CHIESA GIUSEPPINA LTEE03000P CISTERNA 2 CISTERNA 49 14 DI GREGORIO LUCIA LTEE020004 APRILIA 1 APRILIA 49 15 DI MAIO ALESSANDRA LTEE020004 APRILIA 1 APRILIA 49 16 DI NUCCI LUISA LTEE09000A SABAUDIA SABAUDIA 49 17 DI PIRO MARCO LTTD01000R G. FILANGIERI FORMIA 49 18 DI VEGLIA SANDRA LTRI010006 E. FERMI FORMIA 49 19 D'URSO FRANCESCA LTRH01000P A. CELLETTI FORMIA 49 20 FAVOCCIA ANGELA LTEE04300R FORMIA 1 FORMIA 49 21 FICO MARIA FORMIA 49 22 FOROGHI BILAND KIUMARS LTIS01100B IS VITRUVIO POLLIONE FORMIA 49 23 FRANCIOSA MARIA LTTD01000R G. FILANGIERI FORMIA 49 24 GONNELLA LIDIA LTEE020004 APRILIA 1 APRILIA 49 25 LA ROCCA FRANCA LTTD04000L ARTURO BIANCHINI TERRACINA 49 SEDE: IPIA FERMI - VIA E. -

Bollettino 16Gen21
SARS COVID-19: bollettino del 16/01/2021 DRIVE-IN ATTIVI COMUNE INDIRIZZO ORARI Aprilia Fiera (mercato dei fiori) località 8:30 - 13:30 dal lunedì al sabato Campoverde Km 46,600 (direzione Latina) della SS.148 Pontina Gaeta ex Ospedale Monsignor Don Luigi di 9:00 - 15:00 dal lunedì al sabato Liegro via salita cappuccini LATINA ex Istituto SANI- Salvemini viale le 9:00 - 18:30 dal lunedì al sabato Corbusier 393 9:00 - 13:00 la domenica Priverno Casa della Salute 8:30 - 13:30 dal lunedì al sabato via Madonna delle Grazie L'accesso è regolato da prenotazione obbligatoria che dovrà essere effettuata almeno 24 h prima, ad eccezione dei: - Cittadini che devono partire per Stati Esteri, per i quali sarà sufficiente mostrare al personale del “drive-in” idoneo titolo di viaggio e ricevuta di pagamento; - Cittadini contattati dal Dipartimento di Prevenzione, che procederà direttamente ad effettuare la prenotazione. La prenotazione deve essere effettuata tramite registrazione al link https://concorsi.ausl.latina.it/covid19/drivein.jsf , oppure utilizzando QR code di seguito riportato: N.B. Per prenotarsi è necessaria la ricetta prodotta dal proprio medico di Medicina Generale, la prestazione è a carico del Sistema sanitario Nazionale. Gli unici tamponi a pagamento sono quelli richiesti per motivi di viaggio o lavoro. SCREENING PER RICERCA CORONAVIRUS DEDICATI AI RAGAZZI DELLE SCUOLE SUPERIORI Sono rivolti ai giovani residenti nel Distretto 2 della Asl di Latina. E’ obbligatoria la prenotazione tramite il sito della Asl. Sul portale aziendale saranno inoltre disponibili i moduli per il consenso informato da scaricare, compilare e firmare dal genitore nel caso di studenti minori di età. -

Centro Sttorico
Teerrittoriio Poolitica Teerriitoriio Il Fatto TTerritoorio Anche le api Ci vorrebbe una Ancora cemento I famosi antipasti di mia moglie Un’antenna nel loro piccolo ... rivoluzione (liberale) sul territorio Vacanze Pontine a Mezzomonte di Ape Ronzante di Alessandro Petti di Marco Omizzolo di E. Dantes e Roderigo di Cristian Lamagna a pagg. 3 a paag. 5 a pag. 7 a pagg. 8-9 a pag. 11 ENTRO TORICO C S BIMESTRALE GRATUITO - ANNO 6 N. 33 - NOVEMBRE-DICEMBRE 2008 ASSOCIAZIONE CULTURALE “IL CENTRO STORICO” SAN FELICE CIRCEO di ALESSANDRO CRESTI SOOLO CONNTRO TUTTTI Avaritia et adrogantia praecipua validiorum vitia L’avarizia e l’arroganza Mario Vittorio Capponi sono i vizi principali dei potenti “Il tribuno della plebe” se ne è andato! Prepotenza al potere rima di trasferirmi a vivere a San a ale Felice (quasi un quarto di secolo opo diverso tempo di contrasti tra Pfa!) avevo sentito parlare di Vittorio opposizione e maggioranza, que- solo durante i miei soggiorni estivi, quan- d ditori E Dst’ultima (PdL) il 14 novembre u.s. ha do in piazza si formavano ancora capan- prepotentemente rifiutato il candidato pro- nelli di cittadini appassionati alle vicende posto dal PD, Leoluca Orlando, e ha eletto il Se- natore PD, Riccardo Villari, Presidente della del paese. Le opinioni su di lui erano dis- Commissione di Vigilanza sulla RAI, infischian- cordanti, e quando l’argomento si spo- dosene della prassi parlamentare per cui da stava sugli Usi Civici, si formavano due sempre questa scelta spetta all’opposizione. fazioni: una che stravedeva per lui e l’al- Una prepotenza che si commenta da sola, at- tra che lo detestava, ma tutte e due gli ri- tuata praticamente in soli quindici minuti, con un vero e proprio blitz. -

Le Golfe Gaeta
AZIENDA DI PROMOZIONE TURISTICA DELLA PROVINCIA DI LATINA SOMMARIO IL GOLFO DI GAETA Informazioni p. 1 Come arrivare p. 2 Il Golfo di Gaeta p. 4 Le località p. 8 Le Isole Pontine p. 22 Sport e tempo libero p. 24 Stabilimenti balneari p. 26 Locali notturni e ritrovi tipici p. 27 Folklore e manifestazioni p. 28 Gastronomia p. 30 Ambiente e natura p. 32 Per informazioni sull’area e sulle località contenute nella pubblicazione, rivolgersi ai seguenti uffici turistici: Pour tous reinsegnements sur la zone et sur les localités présentées dans cette publication, s’adresser aux bureaux de tourisme suivantes: A.P.T. – Azienda di Promozione Turistica della Provincia di Latina Via Legnano, 42 04100 LATINA – Tel. 0773695404 – Fax 0773661266 www.aptlatinaturismo.it e www.latinaturismo.it - e-mail: [email protected] Ufficio Informazioni: Piazza del Popolo – Tel. 0773480672 I.A.T. – Uffici Informazioni e Assistenza al Turista - Bureaux de renseignements Ideazione Formia Pier Giacomo Sottoriva Viale Unità d’Italia, 30 – 34 (C.A.P. 04023) Tel. 0771771490 - Fax 0771323275 Testi Gaeta Maria Caterina Santini Via E. Filiberto, 5 (C.A.P. 04024) – Tel. 0771461165 – Fax 0771450779 Foto Minturno–Scauri Archivio APT Latina, P. Petrignani, Via Lungomare, 32 (C.A.P. 04028) – Tel. 0771683788 – Fax 0771620829 P. Silvano, L. Orilia, Terracina S. Vannini, Archivio IGDA Via Leopardi (C.A.P. 04019) – Tel. 0773727759 – Fax 0773721173 Coordinamento redazionale Bruno Maragoni, Anna Maria ORGANISMI TURISTICI DI CATEGORIA/ORGANISMES TOURISTIQUES DE CATÉGORIE Mantione Associazione Provinciale Albergatori – c/o Confcommercio Grafica Via dei Volsini, 46 – 04100 Latina – Tel. 0773610678 Alberto Costa, Marco Stingo Traduzioni Confcommercio Latina – Via dei Volsini, 46 – 04100 Latina – Tel. -

Comunicato Stampa
DIREZIONE GENERALE COMUNICATO STAMPA Rispetto alla giornata di ieri, si registrano 42 nuovi casi positivi, distribuiti nei Comuni di Aprilia (13), di Cisterna di Latina (5), di Cori (8), di Formia (2), di Latina (7), di Pontinia (1), di Sermoneta (1), di Sezze (1), di Sonnino (3) e di Terracina (1). Si è registrato il decesso di un paziente, residente nel Comune di Norma. Casi Prevalenza Guariti Deceduti Positivi di cui a domicilio 1644 28,58 682 41 921 811 Si ricorda che, in relazione alla situazione epidemiologica in essere localmente, sono stati attivati nei Comuni di Aprilia e Priverno, locali postazioni permanenti di "Drive in" per l’esecuzione del test antigenico o molecolare volto alla rilevazione del coronavirus SARS-COV2. GAETA: attivo dalle ore 9:00 alle ore 15:00 dal lunedì al sabato c/o ex Ospedale Monsignor Don Luigi di Liegro via salita cappuccini APRILIA : attivo dalle ore 9:00 alle ore 14:00 dal lunedì al sabato c/o Fiera (mercato dei fiori) località Campoverde Km 46,600 (direzione Latina) della SS.148 Pontina PRIVERNO: attivo dalle ore 8:30 alle ore 13:30 dal lunedì al sabato c/o la Casa della Salute sita in via Madonna delle Grazie L'accesso al “drive in” è regolato da prenotazione che dovrà essere effettuata almeno 24 h prima. Pertanto non sarà garantito il tampone alle persone prive di prenotazione, ad eccezione dei seguenti cittadini che potranno presentarsi senza prenotazione: - Cittadini che devono partire per Stati Esteri, dove viene richiesta idonea certificazione, per i quali sarà sufficiente mostrare al personale del “drive in” idoneo titolo di viaggio e ricevuta di pagamento; - Cittadini contattati dal Dipartimento di Prevenzione, che procederà direttamente ad effettuare la prenotazione. -

AMBITO TERRITORIALE DI CACCIA LT-2 Corso Vittorio Emanuele,10 – 04020 MONTE SAN BIAGIO (LT) Tel./Fax 0771-567001 – [email protected]
AMBITO TERRITORIALE DI CACCIA LT-2 Corso Vittorio Emanuele,10 – 04020 MONTE SAN BIAGIO (LT) Tel./Fax 0771-567001 www.atclatina2.it – [email protected] DISCIPLINARE CACCIA AL CINGHIALE STAGIONE 2015/2016 Art. 1 Il presente disciplinare regola l’attività venatoria al cinghiale su tutto il territorio dell’ATC LT2 in base alla Determina Dirigenziale n. 890 del 3 set. 2015, da intendersi integralmente richiamata. L’obiettivo è di raggiungere e mantenere una densità compatibile con le esigenze di salvaguardia delle colture agricole, di tutela delle altre specie selvatiche, per consentire il prelievo in sicurezza, nel rispetto delle norme vigenti. Art. 2 La caccia al cinghiale può essere pertanto, esercitata secondo le seguenti modalità operative: a) battuta o braccata; b) singolarmente, alla cerca. Art. 3 Il Territorio dell’ATC è suddiviso in 4 Comprensori di “Gestione del Cinghiale” aventi dimensioni di carattere intercomunale, suddivisi in 32 “Punti Presa” di carattere comunale e/o intercomunale, destinati alla caccia in battuta o braccata. Ogni “Punto Presa” rappresenta una zona “vocata” per la caccia al Cinghiale (cartografia pubblicata sul sito istituzionale dell’ATC (www.atclatina2.it). Art. 4 I Comprensori di gestione (compresi dei “punti presa” per la caccia in braccata e dei Componenti dell’Assemblea), sono così ripartiti: COMPRENSORI DI GESTIONE E RELATIVA PUNTI PRESA ASSEMBLEA CASTELFORTE – SPIGNO SATURNIA - 1 – Valle Longa (Castelforte) SS.COSMA – MINTURNO 2 – Valle di Suio (Castelforte) Assemblea del Comprensorio: 3 – Pozzari (Castelforte) MASTRILLO Franco - SANTILLI Giuseppe 4 - Macchia Nera (Minturno); SOMMA Luigi – FALSO Massimo 5 - Cerri Aprano ( SS. Cosma e Damiano); CAMPOCHIARO Umberto 6 - (revocato) 7 – Capodacqua (Spigno Saturnia) - Referente: Franco MASTRILLO - Vice: Massimo FALSO ITRI – FORMIA - GAETA 8 - Il Carrubo (Formia) Assemblea del Comprensorio: 9 - Vate Cancello (Formia) FORCINA Franco – SUPINO Franco - DI 10 – Sugherete (Formia) BIASE Benito - SACCOCCIO Daniele 11 - Casa Capraia (Itri) - Referente: Benito DI BIASE 12 – S.