Identifying Repaired Shell Damage and Abnormal Calcification in the Stout Razor Clam Tagelus Plebeius As a Tool to Investigate Its Ecological Interactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Impact of Hydraulic Blade Dredging on a Benthic Megafaunal Community in the Clyde Sea Area, Scotland
Journal of Sea Research 50 (2003) 45–56 www.elsevier.com/locate/seares The impact of hydraulic blade dredging on a benthic megafaunal community in the Clyde Sea area, Scotland C. Hauton*, R.J.A. Atkinson, P.G. Moore University Marine Biological Station Millport (UMBSM), Isle of Cumbrae, Scotland, KA28 0EG, UK Received 4 December 2002; accepted 13 February 2003 Abstract A study was made of the impacts on a benthic megafaunal community of a hydraulic blade dredge fishing for razor clams Ensis spp. within the Clyde Sea area. Damage caused to the target species and the discard collected by the dredge as well as the fauna dislodged by the dredge but left exposed at the surface of the seabed was quantified. The dredge contents and the dislodged fauna were dominated by the burrowing heart urchin Echinocardium cordatum, approximately 60–70% of which survived the fishing process intact. The next most dominant species, the target razor clam species Ensis siliqua and E. arcuatus as well as the common otter shell Lutraria lutraria, did not survive the fishing process as well as E. cordatum, with between 20 and 100% of individuals suffering severe damage in any one dredge haul. Additional experiments were conducted to quantify the reburial capacity of dredged fauna that was returned to the seabed as discard. Approximately 85% of razor clams retained the ability to rapidly rebury into both undredged and dredged sand, as did the majority of those heart urchins Echinocardium cordatum which did not suffer aerial exposure. Individual E. cordatum which were brought to surface in the dredge collecting cage were unable to successfully rebury within three hours of being returned to the seabed. -

MOLLUSCS Species Names – for Consultation 1
MOLLUSCS species names – for consultation English name ‘Standard’ Gaelic name Gen Scientific name Notes Neologisms in italics der MOLLUSC moileasg m MOLLUSCS moileasgan SEASHELL slige mhara f SEASHELLS sligean mara SHELLFISH (singular) maorach m SHELLFISH (plural) maoraich UNIVALVE SHELLFISH aon-mhogalach m (singular) UNIVALVE SHELLFISH aon-mhogalaich (plural) BIVALVE SHELLFISH dà-mhogalach m (singular) BIVALVE SHELLFISH dà-mhogalaich (plural) LIMPET (general) bàirneach f LIMPETS bàirnich common limpet bàirneach chumanta f Patella vulgata ‘common limpet’ slit limpet bàirneach eagach f Emarginula fissura ‘notched limpet’ keyhole limpet bàirneach thollta f Diodora graeca ‘holed limpet’ china limpet bàirneach dhromanach f Patella ulyssiponensis ‘ridged limpet’ blue-rayed limpet copan Moire m Patella pellucida ‘The Virgin Mary’s cup’ tortoiseshell limpet bàirneach riabhach f Testudinalia ‘brindled limpet’ testudinalis white tortoiseshell bàirneach bhàn f Tectura virginea ‘fair limpet’ limpet TOP SHELL brùiteag f TOP SHELLS brùiteagan f painted top brùiteag dhotamain f Calliostoma ‘spinning top shell’ zizyphinum turban top brùiteag thurbain f Gibbula magus ‘turban top shell’ grey top brùiteag liath f Gibbula cineraria ‘grey top shell’ flat top brùiteag thollta f Gibbula umbilicalis ‘holed top shell’ pheasant shell slige easaig f Tricolia pullus ‘pheasant shell’ WINKLE (general) faochag f WINKLES faochagan f banded chink shell faochag chlaiseach bhannach f Lacuna vincta ‘banded grooved winkle’ common winkle faochag chumanta f Littorina littorea ‘common winkle’ rough winkle (group) faochag gharbh f Littorina spp. ‘rough winkle’ small winkle faochag bheag f Melarhaphe neritoides ‘small winkle’ flat winkle (2 species) faochag rèidh f Littorina mariae & L. ‘flat winkle’ 1 MOLLUSCS species names – for consultation littoralis mudsnail (group) seilcheag làthaich f Fam. -

Clams” Fauna Along French Coasts
Asian Journal of Research in Animal and Veterinary Sciences 1(1): 1-12, 2018; Article no.AJRAVS.39207 The Regulation of Interspecific Variations of Shell Shape in Bivalves: An Illustration with the Common “Clams” Fauna along French Coasts Jean Béguinot1* 1Biogéosciences, UMR 6282, CNRS, Université Bourgogne Franche-Comté, 6, Boulevard Gabriel, 21000 Dijon, France. Author’s contribution The sole author designed, analyzed, interpreted and prepared the manuscript. Article Information DOI: 10.9734/AJRAVS/2018/39207 Editor(s): (1) Andras Fodor, Department of Animal Sciences, Ohio State University, USA. Reviewers: (1) Mahmoud Abdelhamid Dawood, Kafrelsheikh University, Egypt. (2) Mbadu Zebe Victorine, Democratic Republic of Congo. Complete Peer review History: http://www.sciencedomain.org/review-history/23116 Received 24th November 2017 th Original Research Article Accepted 6 February 2018 Published 10th February 2018 ABSTRACT I report an unexpected negative covariance occurring between two major parameters governing shell growth in marine bivalves, especially within the order Veneroida. This relationship is highlighted, here, considering a set of forty, rather common species of clams collected from French coasts. Interestingly, this negative covariance has two (geometrically related) consequences on the pattern of variation of shell shape at the inter-specific level: (i) An extended range of variation of shell elongation ‘E’ is made compatible with. (ii) A severely restricted range of variation of the ventral convexity ‘K’ of the shell contour. I suggest that: (i) The extended range of interspecific variation of the shell elongation ‘E’ results from a trend towards larger differentiation between species according to this functionally important parameter E, while, in contrast, (ii) The strongly restricted range of variation of the ventral convexity ‘K’ of the shell contour might arguably result from a common need for improved shell resistance, face to mechanical solicitations from the environment, either biotic or abiotic. -

On the Ciliary Mechanisms and Interrelationships of Lamellibranchs
On the Ciliary Mechanisms and Interrelationships of Lamellibranchs. PAET IV: Cuticular Fusion, with special reference to the Fourth Aperture in certain Lamelli- branchs. By Daphne Atkins, B.Sc. Marine Biological Laboratory, Plymouth. With 11 Text-figures. CONTENTS. PAGE INTRODUCTION 423 HISTOLOGICAL STRUCTURE OF THE JUNCTION .... 424 CUTICULAK FUSION IN RELATION TO THE FOURTH APERTURE IN CERTAIN LAMELLIBRANCHS ....... 436 Origin of the Fourth Aperture ....... 442 SUMMARY ........... 444 INTBODUCTION. A PECULIAR form of fusion, involving the cuticle only, has been found in a number of Lamellibranchs, but has been chiefly studied in the Solenidae. The positions so far discovered in which this type of fusion occurs are between the dorsal edges of the ascending lamellae of the outer demibranchs and the mantle or the visceral mass in Solen marginatus Montagu (=vagina), Ensis siliqua (L.), Ensis arcuatus (Jeffreys), Cultellus pellucidus (Pennant), Solecurtus scopula (Turton) (=candidus), Lutraria lutraria (L.) (=elliptica), and Tellina tenuis da Costa; between the dorsal edges of the ascending lamellae of the two inner demibranchs in Barnea parva (Pennant); and between the mantle lobes in the region between the pedal and fourth apertures in Ensis siliqua, Ensis arcuatus, and Cultellus pellucidus. 424 D. ATKINS HlSTOLOGICAL STRUCTURE OF THE JUNCTION. Material was fixed in Bouin-Duboscq's fluid with the following formula: saturated picric acid in 90 per cent, alcohol, 2 parts; saturated corrosive sublimate (water), 3 parts; 40 per cent, formalin, 1 part; glacial acetic acid, 2 parts. The stains used were Heidenhain's iron haematoxylin counterstained with acid m.f TEXT-FIG. 1 A. Transverse section showing the method of junction of the mantle lobes in the mid-ventral region between the pedal and fourth apertures. -

Assessing the Conservation Status of Marine Habitats: Thoughts from a Sandflat on the Isles of Scilly
MURDOCH RESEARCH REPOSITORY This is the author’s final version of the work, as accepted for publication following peer review but without the publisher’s layout or pagination. The definitive version is available at http://dx.doi.org/10.1016/j.seares.2014.07.013 Warwick, R.M. and Somerfield, P.J. (2014) Assessing the conservation status of marine habitats: thoughts from a sandflat on the Isles of Scilly. Journal of Sea Research, 98. pp. 109-119. http://researchrepository.murdoch.edu.au/23345/ Copyright: © 2014 Elsevier B.V. It is posted here for your personal use. No further distribution is permitted. ÔØ ÅÒÙ×Ö ÔØ Assessing the conservation status of marine habitats: Thoughts from a sandflat on the Isles of Scilly R.M. Warwick, P.J. Somerfield PII: S1385-1101(14)00136-1 DOI: doi: 10.1016/j.seares.2014.07.013 Reference: SEARES 1277 To appear in: Journal of Sea Research Received date: 21 February 2014 Revised date: 14 July 2014 Accepted date: 20 July 2014 Please cite this article as: Warwick, R.M., Somerfield, P.J., Assessing the conservation status of marine habitats: Thoughts from a sandflat on the Isles of Scilly, Journal of Sea Research (2014), doi: 10.1016/j.seares.2014.07.013 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. -
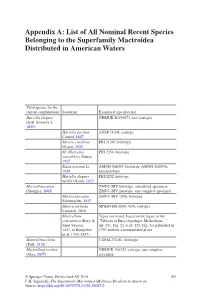
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Iv. Hasil Dan Pembahasan
IV. HASIL DAN PEMBAHASAN 4.1 Hasil 4.1.1 Jenis Bivalvia yang Ditemukan Bivalvia yang ditemukan pada kawasan mangrove Sungai Perpat diperoleh sebanyak 183 individu terdiri dari sembilan famili dan dua belas spesies. Kawasan mangrove Sungai Bunting diperoleh sebanyak 82 individu terdiri dari enam famili dan tujuh spesies. Pada stasiun 1 ditemukan sebanyak empat spesies dengan jumlah 83 individu, stasiun 2 ditemukan sebanyak tiga spesies dengan jumlah 31 individu dan pada stasiun 3 ditemukan sebanyak sembilan spesies dengan jumlah 69 individu. Pada stasiun 4 ditemukan sebanyak satu spesies dengan jumlah 22 individu, stasiun 5 ditemukan sebanyak empat spesies dengan jumlah 52 individu dan pada stasiun 6 ditemukan sebanyak dua spesies dengan jumlah 8 individu. Data jumlah bivalvia yang ditemukan disajikan pada Tabel 3. Tabel 3 Spesies Bivalvia yang ditemukan pada Lokasi Penelitian Famili Nama Lokal Nama Ilmiah Stasiun St 1 St 2 St 3 St 4 St 5 St 6 Arcidae Kerang Bulu Anadara 0 0 2 0 0 3 gubernaculum Veneridae Kerang Anomalodiscus 0 0 1 0 0 0 Pencong squamosus Kerang Mactra grandis 0 0 8 0 0 0 Manis Kerang Meretrix meretrix 0 0 31 0 0 0 Kepah Kedimul Placamen 0 0 3 0 0 5 chloroticum Periplomatidae Kerang Cochlodesma 0 0 3 0 0 0 Lentera praetenue Cyrenidae Lokan Geloina expansa 23 25 0 22 0 0 Isognomonidae Tiram Pohon Isognomon alatus 0 0 2 0 11 0 Mytilidae Kerang Lithophaga teres 19 4 13 0 5 0 Jubing Mactridae Kijing Lutraria lutraria 31 0 0 0 0 0 Pharidae Kerang Pharella javanica 10 2 0 0 21 0 Solenidae Kerang Solen sicarius 0 0 6 0 15 0 Bambu -

NWIFCA Byelaw 3 Or NWIFCA Restrictions on the Use of a Dredge Byelaw 2017;
ANNEX B PAPER TO TSB NOVEMBER 2020 with amended table 1 and minor textual changes MANAGEMENT OF UNREGULATED HAND-GATHERING OF BIVALVES AT LEASOWE BEACH, WIRRAL Purpose: To provide a detailed report to members on the issue of clam gathering at Leasowe Recommendation: i. to implement a permanent byelaw that prohibits the removal of any bivalves from the beach at Leasowe unless under a permit issued under NWIFCA Byelaw 3 or NWIFCA Restrictions on the use of a dredge byelaw 2017; ii. that scientific survey should continue over the next three years to provide data on population recovery in order to inform the byelaw review after 3 years. Situation 2013 - 2020 1. NWIFCA has been monitoring activity levels of hand-gathering of unregulated bivalves on the North Wirral Foreshore since 2013. Significant numbers of gatherers from a variety of ethnic backgrounds, identified as appearing to be mainly Far East / South East Asian (IC5*), had been observed by enforcement officers on the larger spring tides. There were fears that gathering could be commercial and might be causing problems to the sustainability of the stocks. 2. As regularly reported to the Authority, increased effort in patrolling the area and recording sightings showed that the activity only occurred during the largest spring tides in the summer, (see Table 1) and that it was being carried out by family groups gathering clams for personal consumption. This was corroborated through liaison with Environmental Health Officers and other enforcement agencies, through sharing of intelligence and joint-working, and no evidence was found to suggest the catch was being sold. -

Cancer Pagurus
Cancer pagurus Cancer pagurus, commonly known as the edible crab or The first pereiopod is modified into a strong cheliped brown crab, is a species of crab found in the North Sea, (claw-bearing leg): the claw’s fingers, the dactylus and North Atlantic Ocean and perhaps in the Mediterranean propodus, are black at the tips.[1] The other pereiopods Sea. It is a robust crab of a reddish-brown colour, hav- are covered with rows of short stiff setae; the dactylus of ing an oval carapace with a characteristic “pie crust” edge each is black towards the tip, and ends in a sharp point.[1] and black tips to the claws. A mature adult may have a From the front, the antennae and antennules are visi- carapace width of up to 25 cm (10 in) and weigh up to 3 ble. Beside these there are the orbits in which the eyes kg (6.6 lb). C. pagurus is a nocturnal predator, targeting are situated.[4] The mouthparts comprise three pairs of a range of molluscs and crustaceans. It is the subject of maxillipeds, behind which there are a pair of maxillae, a the largest crab fishery in Western Europe, centred on the pair of maxillules, and finally the mandibles.[4] coasts of the British Isles, with more than 60,000 tonnes caught annually. In common with most crabs, the abdomen is folded under the thorax and shows clear sexual dimorphism: in males it is comparatively narrow, whereas in the female it is [4] 1 Description wider. 2 Life cycle Reproduction occurs in winter; the male stands over the female and forms a cage with his legs protecting her while [2] Mouthparts and chelae of a female she moults. -

Tidal Pools 22
TIDAL POOLS LEGEND 1. DAY SHALLOW - BELOW LOW TIDE 1. Edible Crab Cancer pagurus 2. Great Scallop Pecten maximus 3. Light Bulb Sea Squirt Clavelina lepadiformis 4. Feather Star Antedon bifida 5. Cushion Star Porania pulvillus 6. Plaice Pleuronectes platessa 7. Common Sea Urchin Echinus esculentus 8. Corkwing Wrasse Crenilabrus melops 9. Octopus Octopus vulgaris 10. Snake Pipefish Entelurus aequoreus 11. Bootlace Weed or Mermaid’s Tresses Chorda filum 12. Cuvie or Forest Kelp Laminaria hyperborea 2. DAY BETWEEN HIGH AND LOW TIDE 1. Crawfish Palinurus elephas 2. Sea Slug Doto fragilis 3. Mussels Mytilus edulis 4. Sea Potato Echinocardium cordatum 5. Common Starfish Asterias rubens 6. Gutweed Enteramorpha intestinalis 7. Sea Lemon Archidoris pseudoaogus 8. Sand Eel Ammodytes tobianus 9. Lesser Spotted Dogfish Scyliorhinus canicula 10. Edible Cockle Cerastoderma edule 11. Hermit Crab Pagurus bernhardus 12. Sea Cucumber Pawsonia saxicola 13. Oysters Ostrea edulis 3. DAY ABOVE HIGH TIDE SEASHORE 1. Ragworm Nereis diversicolor 2. Tower Shell Turritella communis 3. Common Piddock Pholas dactylus 4. Ship Worm Teredo 5. Empty Oyster Shell Ostrea edulis 6. Hairy Crab Pilumnus hirtellus 7. Common Acorn Barnacle Balanus balanoides 8. Common Lizard Lacerta vivipara 9. Common Dog Whelk Buccinum undatum 10. Chitons Lepidopleurus asellus 11. Lichen Xanthoria parietina 12. Common Nutshell Nucula nucleus 13. Keel Worm Pomatoceros triqueter 14. Sand Hopper Talitrus saltator 15. Sea Holly Eryngium maritimum 16. Dogfish Egg Purse (Mermaid’s Purse) Scyliorhinus canicula 17. Bootlace Weed Chorda filum 18. Cockle Shell Cerastoderma edule 19. Periwinkle Littorina litterea 20. Common Otter Shell Lutraria lutraria 4. ROCK POOL 1. Lobster Homarus gammarus 2. -

Edible Crab (Cancer Pagurus)
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Edible crab (Cancer pagurus) MarLIN – Marine Life Information Network Marine Evidence–based Sensitivity Assessment (MarESA) Review Ken Neal & Emily Wilson 2008-05-08 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1179]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Neal, K.J. & Wilson, E. 2008. Cancer pagurus Edible crab. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1179.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2008-05-08 Edible crab (Cancer pagurus) - Marine Life Information Network See online review for distribution map Cancer pagurus, edible crab. -
Shell Identification Guide
Version of February 2012. Prepared by Ian Wallace, World Museum, for the Liverpool Bay Marine Recording Partnership 1 Shell pages These supplementary notes are designed to be used in conjunction with the recording sheets Liverpool Bay Marine Recording Partnership Additional help identifying difficult shells and those not covered by the recording sheets Designed for recording from beaches between Fleetwood and Colwyn Bay Version of February 2012. Prepared by Ian Wallace, World Museum, for the Liverpool Bay Marine Recording Partnership 2 Index to main sections Page 6 Necklace Shells 8 Winkles 10 Whelks 12 Hydrobia and similar 13 vagrants from dune and saltmarsh 15 Oysters 17 Mussels 18 Cockles 20 Scallops 21 Razors 27 Otters and Gapers (young shells page 45, 51 & 52) 29 Piddocks 31 Tellins (also 34, 48 and 51) 34 Sunset Shell start at 37 for general white bivalves such as 39 Artemis Shells 41 – 44 & 48 Trough Shells 51 Furrow Shells 45, 51 & 52 young Gapers & Otters 50 Nut Shells 54 assorted (also 3 & 4) Version February 2012. Prepared by Ian Wallace, World Museum, for the Liverpool Bay Marine Recording Partnership 3 Preliminary Note Why are some shells black? It is not oil pollution, or pollution of any sort, it is quite natural There is a lot of iron oxide, or rust, on sand grains, that comes from the rocks they were made from. That is why most beaches are sandy coloured. There are also millions of bacteria living in the sand. If oxygenated water cannot get down to the lower layers of sand, perhaps because the sand-grains are very small or there is a lot of mud too, then strange things happen! The bacteria feed on the organic matter that is abundant in all beach sand.