Experimental Studies of the Mechanisms Producing Hypocalcemia in Hypernatremic States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyponatremia and Hypernatremia MICHAEL M
This is a corrected version of the article that appeared in print. Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia MICHAEL M. BRAUN, DO, Madigan Army Medical Center, Tacoma, Washington CRAIG H. BARSTOW, MD, Womack Army Medical Center, Fort Bragg, North Carolina NATASHA J. PYZOCHA, DO, Madigan Army Medical Center, Tacoma, Washington Hyponatremia and hypernatremia are common findings in the inpatient and outpatient settings. Sodium disorders are associated with an increased risk of morbidity and mortality. Plasma osmolality plays a critical role in the patho- physiology and treatment of sodium disorders. Hyponatremia and hypernatremia are classified based on volume status (hypovolemia, euvolemia, and hypervolemia). Sodium disorders are diagnosed by findings from the history, physical examination, laboratory studies, and evaluation of volume status. Treatment is based on symptoms and underlying causes. In general, hyponatremia is treated with fluid restriction (in the setting of euvolemia), isotonic saline (in hypovolemia), and diuresis (in hypervolemia). A combination of these therapies may be needed based on the presentation. Hypertonic saline is used to treat severe symptomatic hyponatremia. Medications such as vaptans may have a role in the treatment of euvolemic and hypervolemic hyponatremia. The treatment of hypernatremia involves correcting the underlying cause and correcting the free water deficit. Am( Fam Physician. 2015;91(5):299-307. Copy- right © 2015 American Academy of Family Physicians.) More online yponatremia is a common elec- a worse prognosis in patients with liver cir- at http://www. trolyte disorder defined as a rhosis, pulmonary hypertension, myocardial aafp.org/afp. serum sodium level of less than infarction, chronic kidney disease, hip frac- CME This clinical content 135 mEq per L.1-3 A Dutch sys- tures, and pulmonary embolism.1,8-10 conforms to AAFP criteria Htematic review of 53 studies showed that the for continuing medical Etiology and Pathophysiology education (CME). -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -

Novel Mutations Associated with Inherited Human Calcium-Sensing
1 180 A García-Castaño, Novel mutations in the calcium 180:1 59–70 Clinical Study L Madariaga and others receptor gene Novel mutations associated with inherited human calcium-sensing receptor disorders: A clinical genetic study Alejandro García-Castaño1,*, Leire Madariaga1,2,*, Gustavo Pérez de Nanclares1,2, Gema Ariceta3, Sonia Gaztambide1,2 and Luis Castaño1,2 on behalf of Spanish Endocrinology Group and Renal Tube Group Correspondence should be addressed 1Biocruces Bizkaia Health Research Institute, CIBERDEM, CIBERER, Barakaldo, Spain, 2Hospital Universitario Cruces, to L Castaño UPV/EHU, Barakaldo, Spain, and 3Hospital Universitario Materno-Infantil Vall d’Hebron, Autonomous University of Email Barcelona, Barcelona, Spain LUISANTONIO. *(A García-Castaño and L Madariaga contributed equally to this work) CASTANOGONZALEZ@ osakidetza.eus Abstract Objective: Molecular diagnosis is a useful diagnostic tool in calcium metabolism disorders. The calcium-sensing receptor (CaSR) is known to play a central role in the regulation of extracellular calcium homeostasis. We performed clinical, biochemical and genetic characterization of sequence anomalies in this receptor in a cohort of 130 individuals from 82 families with suspected alterations in the CASR gene, one of the largest series described. Methods: The CASR gene was screened for mutations by polymerase chain reaction followed by direct Sanger sequencing. Results: Presumed CaSR-inactivating mutations were found in 65 patients from 26 families. These patients had hypercalcemia (median: 11.3 mg/dL) but normal or abnormally high parathyroid hormone (PTH) levels (median: 52 pg/ mL). On the other hand, presumed CaSR-activating mutations were detected in 17 patients from eight families. These patients had a median serum calcium level of 7.4 mg/dL and hypoparathyroidism (median: PTH 13 pg/mL). -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis
J Clin Res Pediatr Endocrinol 2015;7(Suppl 2):77-92 A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis Yaşar Şen, Sevil Arı Yuca, Fuat Buğrul Blood and urine tests were done in order to find the etiology of hypercalcemia (PTH: 65.5 pg/mL, 25-hydroxy vitamin Selçuk University Faculty of Medicine, Department of Pediatric D: 43.5 ng/mL, urine Ca/Cr ratio 0.19, urine Ca clearance Endorcinology, Konya, Turkey 0.004). In the CaSR gene mutation study, A986S and R990G polymorphisms were heterozygous. 1 month after hydration Introduction: Familial hypocalciuric hypercalcemia (FHH) and clinical state being back to normal, Ca levels were is an autosomal dominant disorder which occurs by an in normal range. In stressful situations, he experienced inactivating gene mutation in the calcium (Ca)-sensing hypernatremia and hypercalcemia together. receptor gene (CaSR). Prevalence is estimated at 1: 78000. Discussion: Polymorphisms may change protein function Ca regulates parathormone (PTH) secretion via CaSR on and capacity of one to repair its damaged DNA. Genetic parathyroid cells. Low levels of Ca increases PTH secretion. polymorphisms help us to define personal sensitivities to CaSR mutation that causes loss of function, leads to total some diseases. The most common CaSR polymorphisms or partial insensitivity of parathyroid cells to Ca’s inhibitory are A986S, R990G, Q1011E and A826T. Our patient effect. For this reason, in order to suppress Ca’s PTH had A986S and R990G mutations. Heterozygous CaSR secretory effect, the setpoint is raised. A higher blood Ca gene mutations generally cause mild disorders in clinic. level is needed to suppress the PTH secretion. -

Non-Accidental Salt Poisoning
448 ArchivesofDiseasein Childhood 1993; 68: 448-452 Non-accidental salt poisoning Roy Meadow Arch Dis Child: first published as 10.1136/adc.68.4.448 on 1 April 1993. Downloaded from Abstract Table I Presentation of12 childrenfrom 10families The clinical features of 12 children who Sex (M/F) 6/6 incurred non-accidental salt poisoning are Age (months) of first confirmed hypernatraemia reported. The children usually presented to 11 children I 5-9 (median 2 - 5) 1 child 41 hospital in the first six months of life with Duration (months) of recurrent unexplained hypernatraemia and associated hypernatraemia 1-45 (median 3) illness. Most ofthe children suffered repetitive poisoning before detection. The perpetrator was believed to be the mother for 10 children, the mother confessed to the poisoning and the father for one, and either parent for one. explained how she had done it.) Four children had serum sodium concentra- tions above 200 mmol/l. Seven children had incurred other fabricated illness, drug inges- PRESENTATION tion, physical abuse, or failure to thrive/ The main features are outlined in table 1. neglect. Two children died; the other 10 Usually the child was presented to hospital remained healthy in alternative care. Features within the first three months of life because of are described that should lead to earlier repetitive illness. Vomiting was the predominant detection of salt poisoning; the importance of feature, often associated with diarrhoea and checking urine sodium excretion, whenever failure to thrive. At times there was drowsiness hypernatraemia occurs, is stressed. which, on occasion, could amount to coma. Four (Arch Dis Child 1993; 68: 448-452) children had markedly abnormal neurological signs including rigidity, hyper-reflexia, and seizures at the time of hypernatraemia. -

051800 Hypernatremia
PRIMARY CARE Review Articles Primary Care clinical interventions or accidental sodium loading (Table 1 and Fig. 1E). Because sustained hypernatremia can occur only when thirst or access to water is impaired, the groups HYPERNATREMIA at highest risk are patients with altered mental status, intubated patients, infants, and elderly persons.12 Hy- HORACIO J. ADROGUÉ, M.D., pernatremia in infants usually results from diarrhea, AND NICOLAOS E. MADIAS, M.D. whereas in elderly persons it is usually associated with infirmity or febrile illness.6,13,14 Thirst impairment also occurs in elderly patients.15,16 Frail nursing home residents and hospitalized patients are prone to hy- HE serum sodium concentration and thus se- pernatremia because they depend on others for their rum osmolality are closely controlled by wa- water requirements.7 ter homeostasis, which is mediated by thirst, T 1 CLINICAL MANIFESTATIONS arginine vasopressin, and the kidneys. A disruption in the water balance is manifested as an abnormality Signs and symptoms of hypernatremia largely re- in the serum sodium concentration — hypernatre- flect central nervous system dysfunction and are prom- mia or hyponatremia.2,3 Hypernatremia, defined as a inent when the increase in the serum sodium con- rise in the serum sodium concentration to a value ex- centration is large or occurs rapidly (i.e., over a period ceeding 145 mmol per liter, is a common electrolyte of hours).1,6 Most outpatients with hypernatremia disorder. Because sodium is a functionally imperme- are either very young or very old.17 Common symp- able solute, it contributes to tonicity and induces the toms in infants include hyperpnea, muscle weakness, movement of water across cell membranes.4 There- restlessness, a characteristic high-pitched cry, insom- fore, hypernatremia invariably denotes hypertonic hy- nia, lethargy, and even coma.5,13 Convulsions are typ- perosmolality and always causes cellular dehydration, ically absent except in cases of inadvertent sodium at least transiently (Fig. -
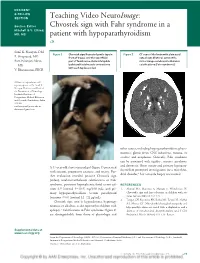
Chvostek Sign with Fahr Syndrome in a Patient with Hypoparathyroidism Sunil K
RESIDENT & FELLOW SECTION Teaching Video NeuroImage: Section Editor Chvostek sign with Fahr syndrome in a Mitchell S.V. Elkind, MD, MS patient with hypoparathyroidism Sunil K. Narayan, DM Figure 1 Chvostek sign: Repeated gentle taps in Figure 2 CT scan of the brain with plain axial P. Sivaprasad, MD front of tragus over the superficial cuts shows bilateral, symmetric, Ram Niranjan Sahoo, part of facial nerve elicits infatigable mirror-image cerebrostriothalamic MD ipsilateral facial muscle contractions calcifications (Fahr syndrome) with each tap (see video) V. Bhuvaneswari, FRCR Address correspondence and reprint requests to Dr. Sunil K. Narayan, Professor and Head of the Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India 605 006 [email protected] [email protected] other causes, including hyperparathyroidism; phaco- matoses; gliosis from CNS infections, trauma, or strokes; and neoplasms. Clinically, Fahr syndrome can be associated with rigidity, seizures, psychosis, and dementia. Short stature and primary hypopara- A 17-year-old short-statured girl (figure 1) presented thyroidism prompted investigations for a mitochon- with seizures, progressive cataracts, and tetany. Fur- drial disorder,2 but a muscle biopsy was normal. ther evaluation revealed positive Chvostek sign (video), cerebrostriothalamic calcifications or Fahr syndrome, persistent hypocalcemia (total serum cal- REFERENCES cium 6.5 [normal 9–10.5] mg/100 mL), and pri- 1. Ahmed MA, Martinez A, Mariam S, Whitehouse W. mary hypoparathyroidism (serum parathyroid Chvostek’s sign and hypocalcaemia in children with sei- hormone Ͻ3.0 [normal 12–72] pg/mL). zures. Seizure 2004;13:217–222. 2. Tengan CH, Kiyomoto BH, Rocha MS, Tavares VL, Gabbai Chvostek sign, seen in hypocalcemia, hypomag- AA, Moraes CT. -

Neurologic Complications of Electrolyte Disturbances and Acid–Base Balance
Handbook of Clinical Neurology, Vol. 119 (3rd series) Neurologic Aspects of Systemic Disease Part I Jose Biller and Jose M. Ferro, Editors © 2014 Elsevier B.V. All rights reserved Chapter 23 Neurologic complications of electrolyte disturbances and acid–base balance ALBERTO J. ESPAY* James J. and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders, Department of Neurology, UC Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA INTRODUCTION hyperglycemia or mannitol intake, when plasma osmolal- ity is high (hypertonic) due to the presence of either of The complex interplay between respiratory and renal these osmotically active substances (Weisberg, 1989; function is at the center of the electrolytic and acid-based Lippi and Aloe, 2010). True or hypotonic hyponatremia environment in which the central and peripheral nervous is always due to a relative excess of water compared to systems function. Neurological manifestations are sodium, and can occur in the setting of hypovolemia, accompaniments of all electrolytic and acid–base distur- euvolemia, and hypervolemia (Table 23.2), invariably bances once certain thresholds are reached (Riggs, reflecting an abnormal relationship between water and 2002). This chapter reviews the major changes resulting sodium, whereby the former is retained at a rate faster alterations in the plasma concentration of sodium, from than the latter (Milionis et al., 2002). Homeostatic mech- potassium, calcium, magnesium, and phosphorus as well anisms protecting against changes in volume and sodium as from acidemia and alkalemia (Table 23.1). concentration include sympathetic activity, the renin– angiotensin–aldosterone system, which cause resorption HYPONATREMIA of sodium by the kidneys, and the hypothalamic arginine vasopressin, also known as antidiuretic hormone (ADH), History and terminology which prompts resorption of water (Eiskjaer et al., 1991). -

Disorders of Sodium and Water Balance
Disorders of Sodium and Water Balance Theresa R. Harring, MD*, Nathan S. Deal, MD, Dick C. Kuo, MD* KEYWORDS Dysnatremia Water balance Hyponatremia Hypernatremia Fluids for resuscitation KEY POINTS Correct hypovolemia before correcting sodium imbalance by giving patients boluses of isotonic intravenous fluids; reassess serum sodium after volume status normalized. Serum and urine electrolytes and osmolalities in patients with dysnatremias in conjunction with clinical volume assessment are especially helpful to guide management. If an unstable patient is hyponatremic, give 2 mL/kg of 3% normal saline (NS) up to 100 mL over 10 minutes; this may be repeated once if the patient continues to be unstable. If unstable hypernatremic patient, give NS with goal to decrease serum sodium by 8 to 15 mEq/L over 8 hours. Correct stable dysnatremias no faster than 8 mEq/L to 12 mEq/L over the first 24 hours. INTRODUCTION Irregularities of sodium and water balance most often occur simultaneously and are some of the most common electrolyte abnormalities encountered by emergency med- icine physicians. Approximately 10% of all patients admitted from the emergency department suffer from hyponatremia and 2% suffer from hypernatremia.1 Because of the close nature of sodium and water balance, and the relatively rigid limits placed on the central nervous system by the skull, it is not surprising that most symptoms related to disorders of sodium and water imbalance are neurologic and can, therefore, be devastating. Several important concepts are crucial to the understanding of these disorders, the least of which include body fluid compartments, regulation of osmo- lality, and the need for rapid identification and appropriate management.