A Review on Metastable Silicon Allotropes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
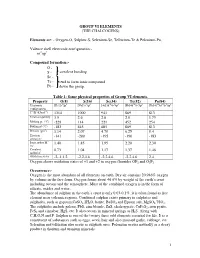
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Silicide-Based Hydrogen Generation for Back-Up Power and Portable Fuel Cells
Silicide-Based Hydrogen Generation for Back-Up Power and Portable Fuel Cells On-demand hydrogen generation systems for high-performance, low-cost and portable fuel cells Hydrogen fuel cells have great promise as a sustainable Fuel Cell System Benefits energy source, but hydrogen generation and storage issues continue to prevent their widespread acceptance. Many portable hydrogen fuel cell systems use hydrogen supply Energy Density Energy systems, such as high-pressure tanks, metal hydrides, and density exceeds 1,000 W-Hrs/kg chemical hydrides, to store needed quantities of hydrogen. These systems have not gained market acceptance, however, Power Density Very fast, due to significant safety risks, difficulties in hydrogen control load following reactivity enables management and, in most cases, the logistical difficulty of high power output without refilling high-pressure tanks. thermal run away. SiGNa Chemistry has taken a different approach to the Control Hydrogen generation is hydrogen generation and storage issues. SiGNa’s solution uses based on the amount of water sodium silicide to produce clean hydrogen gas in real time, as pumped into the NaSi, enabling needed by the fuel cell and at pressures less than those found rapid starts/restarts (>30 sec.) at in a common soda can. SiGNa’s hydrogen-generation process room temperature. is safe, clean and scalable; this revolutionary approach has enabled cost-effective back-up power and portable fuel cell Stability Material is stable over systems that are perfectly suited for the military, medical, all practical temperature ranges (-55 to 300°C) with an unlimited transportation, and electronics industries. storage lifetime. Sodium Silicide Pressure is determined by Sodium silicide (NaSi) and its hybrids are non-flammable, system design, not material air-stable powders that instantly react with water (or water characteristics (<30 psi). -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

The Chemistry of Carbene-Stabilized
THE CHEMISTRY OF CARBENE-STABILIZED MAIN GROUP DIATOMIC ALLOTROPES by MARIHAM ABRAHAM (Under the Direction of Gregory H. Robinson) ABSTRACT The syntheses and molecular structures of carbene-stabilized arsenic derivatives of 1 1 i 1 1 AsCl3 (L :AsCl3 (1); L : = :C{N(2,6- Pr2C6H3)CH}2), and As2 (L :As–As:L (2)), are presented herein. The potassium graphite reduction of 1 afforded the carbene-stabilized diarsenic complex, 2. Notably, compound 2 is the first Lewis base stabilized diatomic molecule of the Group 13–15 elements, in the formal oxidation state of zero, in the fourth period or lower of the Periodic Table. Compound 2 contains one As–As σ-bond and two lone pairs of electrons on each arsenic atom. In an effort to study the chemistry of the electron-rich compound 2, it was combined with an electron-deficient Lewis acid, GaCl3. The addition of two equivalents of GaCl3 to 2 resulted in one-electron oxidation of 2 to 1 1 •+ – •+ – give [L :As As:L ] [GaCl4] (6 [GaCl4] ). Conversely, the addition of four equivalents of GaCl3 to 2 resulted in two- electron oxidation of 2 to give 1 1 2+ – 2+ – •+ [L :As=As:L ] [GaCl4 ]2 (6 [GaCl4 ]2). Strikingly, 6 represents the first arsenic radical to be structurally characterized in the solid state. The research project also explored the reactivity of carbene-stabilized disilicon, (L1:Si=Si:L1 (7)), with borane. The reaction of 7 with BH3·THF afforded two unique compounds: one containing a parent silylene (:SiH2) unit (8), and another containing a three-membered silylene ring (9). -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Optical and Collective Properties of Excitons in 2D Semiconductors
Optical and Collective Properties of Excitons in 2D Semiconductors by Matthew N. Brunetti A dissertation submitted to the Graduate Faculty in Physics in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York 2019 ii © 2019 Matthew N. Brunetti All Rights Reserved iii This manuscript has been read and accepted by the Graduate Faculty in Physics in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy. Professor Oleg L. Berman Date Chair of Examining Committee Professor Igor L. Kuskovsky Date Executive Officer Professor Roman Ya. Kezerashvili Distinguished Professor Godfrey Gumbs Professor Antonios Balassis Professor Paula Fekete Supervisory Committee The City University of New York iv Abstract Optical and Collective Properties of Excitons in 2D Semiconductors by Matthew N. Brunetti Advisers: Professor Oleg L. Berman & Professor Roman Ya. Kezerashvili We study the properties of excitons in 2D semiconductors (2DSC) by numerically solv- ing the Schrödinger equation for an interacting electron and hole in the effective mass ap- proximation, then calculating optical properties such as the transition energies, oscillator strengths, and absorption coefficients. Our theoretical approach allows us to consider both direct excitons in monolayer (ML) 2DSC and spatially indirect excitons in heterostructures (HS) consisting of two 2DSC MLs separated by few-layer insulating hexagonal boron nitride (h-BN). In particular, we study indirect excitons in TMDC HS, namely MoS2, MoSe2, WS2, and WSe2; both direct and indirect excitons in the buckled 2D allotropes of silicon, germa- nium, and tin, known as silicene, germanene, and stanene respectively, or collectively as the Xenes; and both direct and indirect excitons in the anisotropic 2DSC phosphorene, the 2D allotrope of black phosphorus. -

Nasi and Na-SG Powder Hydrogen Fuel Cells
NaSi and Na-SG Powder Hydrogen Fuel Cells Andrew Wallace and Michael Lefenfeld SiGNa Chemistry June 7-11, 2010 www.signachem.com [email protected] ST055 This presentation does not contain any proprietary, confidential, or otherwise restricted information Program Overview Timeline Barriers Start date: 08/01/2008 Enabling Technology for Near-Term High- Volume Fuel Cell Commercialization for End date: 01/31/2011 Portable and Back-up Power Applications Percent complete: 66% Budget Partners FY08 Funding: $1,476,000 University of Texas Austin - CEM FY09 Funding: $951,500 • Reactor Mechanism Research • Richard Thompson and Michael Lewis Trulite Inc. • 250W Fuel Cell Demonstration System • John Patton 2 Technology Overview Relevance • Sodium Silicide rapidly liberates hydrogen from water (or water solutions) leaving a benign common industrial chemical (sodium silicate) 2NaSi(s) + 5H2O(ℓ) 5H2(g) + Na2Si2O5(aq) (No Catalyst, Room Temperature) • Significant System Benefits for Portable Power Applications (1 W to 3 kW) – Safety: Does not ignite or oxidize in air at standard conditions even when fully exposed to air (i.e. opened storage canister). – Thermal Stability: Material is stable over all practical temperature ranges (-55 to 300°C) – Storage: The material has been demonstrated to have a shelf-life of over two years but is capable for being stored for significantly longer – Pressure: The maximum developed pressure is determined by the system design not the material characteristics. The maximum pressure is expected to be a nominal 30 psi (material capable of 1000’s) – Ease of Use: No catalyst required to produce hydrogen gas – By-products: Generates a non-toxic aqueous waste, sodium silicate. -

Silicite: the Layered Allotrope of Silicon
Silicite: the layered allotrope of silicon Seymur Cahangirov,1 V. Ongun Oz¸celik,¨ 2, 3 Angel Rubio,1, ∗ and Salim Ciraci4, y 1Nano-Bio Spectroscopy Group and ETSF Scientific Development Centre, Departamento de F´ısica de Materiales, Universidad del Pa´ısVasco, CSIC-UPV/EHU-MPC and DIPC, Avenida de Tolosa 72, E-20018 San Sebastian, Spain 2UNAM-National Nanotechnology Research Center, Bilkent University, 06800 Ankara, Turkey 3Institute of Materials Science and Nanotechnology, Bilkent University, Ankara 06800, Turkey 4Department of Physics, Bilkent University, Ankara 06800, Turkey (Dated: September 28, 2018) Based on first-principles calculation we predict two new thermodynamically stable layered-phases of silicon, named as silicites, which exhibit strong directionality in the electronic and structural properties. As compared to silicon crystal, they have wider indirect band gaps but also increased absorption in the visible range making them more interesting for photovoltaic applications. These stable phases consistp p of intriguing stacking of dumbbell patterned silicene layers having trigonal structure with 3 × 3 periodicity of silicene and have cohesive energies smaller but comparable to that of the cubic diamond silicon. Our findings also provide atomic scale mechanisms for the growth of multilayer silicene as well as silicites. PACS numbers: 68.65.Ac, 73.61.Ey, 81.05.Dz I. INTRODUCTION II. METHODS We have performed state-of-the-art density functional Early studies1,2 to answer the critical question of theory (DFT) calculations within generalized gradi- ent approximation(GGA). We used projector-augmented whether Si can form graphene-like monolayer structures 14 have been ruled out initially by the arguments that Si wave potentials PAW and the exchange-correlation po- 3{5 tential is approximated with Perdew-Burke-Ernzerhof, does not have a layered allotrope like graphite . -

2. Allotropy in Carbon the Property Due to Which an Element Exists In
SUBJECT-CHEMISTRY DATE- 22/11/2020 CLASS- X TOPICS- Chap. 4 Carbon and its Compounds Carbon is one of the most essential components of living organisms. There are two stable isotopes of carbon C-12 and C-13. After these two one more isotope of carbon is present C-14. Carbon is used for radiocarbon dating One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation. Carbon has many special abilities out of all one unique ability is that carbon forms double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen. These two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms. Allotrope is nothing but the existence of an element in many forms which will have different physical property but will have similar chemical properties and its forms are called allotropes of allotropic forms. Allotropes are defined as the two or more physical forms of one element. These allotropes are all based on carbon atoms but exhibit different physical properties, especially with regard to hardness. The two common, crystalline allotropes of carbon are diamond and graphite. Carbon shows allotropy because it exists in different forms of carbon. Though these allotropes of carbon have a different crystal structure and different physical properties, their chemical properties are the same and show similar chemical properties. Both diamond and graphite have symbol C. Both give off carbon dioxide when strongly heated in the presence of oxygen. -

Intercalation of Si Between Mos2 Layers Rik Van Bremen‡1, Qirong Yao‡1, Soumya Banerjee2, Deniz Cakir2, Nuri Oncel2 and Harold J
Intercalation of Si between MoS2 layers Rik van Bremen‡1, Qirong Yao‡1, Soumya Banerjee2, Deniz Cakir2, Nuri Oncel2 and Harold J. W. Zandvliet*1 Full Research Paper Open Access Address: Beilstein J. Nanotechnol. 2017, 8, 1952–1960. 1Physics of Interfaces and Nanomaterials, MESA+ Institute for doi:10.3762/bjnano.8.196 Nanotechnology, University of Twente, P.O. Box 217, 7500AE Enschede, Netherlands and 2Department of Physics and Received: 29 March 2017 Astrophysics, University of North Dakota, Grand Forks, ND 58202, Accepted: 21 August 2017 USA Published: 19 September 2017 Email: This article is part of the Thematic Series "Silicene, germanene and other Harold J. W. Zandvliet* - [email protected] group IV 2D materials". * Corresponding author ‡ Equal contributors Guest Editor: P. Vogt Keywords: © 2017 van Bremen et al.; licensee Beilstein-Institut. intercalation; molybdenum disulfide; scanning tunneling microscopy; License and terms: see end of document. silicene; two-dimensional materials Abstract We report a combined experimental and theoretical study of the growth of sub-monolayer amounts of silicon (Si) on molybdenum disulfide (MoS2). At room temperature and low deposition rates we have found compelling evidence that the deposited Si atoms intercalate between the MoS2 layers. Our evidence relies on several experimental observations: (1) Upon the deposition of Si on pristine MoS2 the morphology of the surface transforms from a smooth surface to a hill-and-valley surface. The lattice constant of the hill-and-valley structure amounts to 3.16 Å, which is exactly the lattice constant of pristine MoS2. (2) The transitions from hills to valleys are not abrupt, as one would expect for epitaxial islands growing on-top of a substrate, but very gradual. -

Technology Breakthrough Fuels Laptops and Phones, Recharges Scientist's 60-Year Career 17 February 2011
Technology breakthrough fuels laptops and phones, recharges scientist's 60-year career 17 February 2011 How does a scientist fuel his enthusiasm for "I've been working with alkali metals for 50 years," chemistry after 60 years? By discovering a new he said. "My research was closely related to what energy source, of course. SiGNa was looking for. So when they came to me with their idea, it was a relatively easy adaptation to This week, SiGNa Chemistry Inc. unveiled its new make." hydrogen cartridges, which provide energy to fuel cells designed to recharge cell phones, laptops Dye came to MSU in 1953 - two years before MSU and GPS units. The green power source is geared was a university. Based on the products that can be toward outdoor enthusiasts as well as residents of linked to Dye's research just in the last year, it's the Third World, where electricity in homes is clear that he is reaping the rewards of his six considered a luxury. decades of scientific sowing. "SiGNa has created an inherently-safe solution to Using a similar process, Dye was able to assist the produce electric power, resulting in an eco-friendly creation of a fuel source to power electric bicycles. and cost-effective portable solution," said Michael The fuel cell, developed by SiGNa's partners, Lefenfeld, SiGNa's CEO. ranges in size from 1 watt to 3 kilowatts and is capable of pushing a bicycle up to 25 mph for The spark for this groundbreaking technology approximately 100 miles. came from James Dye's Michigan State University laboratory.