Microrna and Messengerrna Expression Changes During Interferon-Beta and Glatiramer Acetate Treatment in Peripheral Blood Cells of Multiple Sclerosis Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Molecular and Genetic Analysis of Otosclerosis
A molecular and genetic analysis of otosclerosis Joanna Lauren Ziff Submitted for the degree of PhD University College London January 2014 1 Declaration I, Joanna Ziff, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Where work has been conducted by other members of our laboratory, this has been indicated by an appropriate reference. 2 Abstract Otosclerosis is a common form of conductive hearing loss. It is characterised by abnormal bone remodelling within the otic capsule, leading to formation of sclerotic lesions of the temporal bone. Encroachment of these lesions on to the footplate of the stapes in the middle ear leads to stapes fixation and subsequent conductive hearing loss. The hereditary nature of otosclerosis has long been recognised due to its recurrence within families, but its genetic aetiology is yet to be characterised. Although many familial linkage studies and candidate gene association studies to investigate the genetic nature of otosclerosis have been performed in recent years, progress in identifying disease causing genes has been slow. This is largely due to the highly heterogeneous nature of this condition. The research presented in this thesis examines the molecular and genetic basis of otosclerosis using two next generation sequencing technologies; RNA-sequencing and Whole Exome Sequencing. RNA–sequencing has provided human stapes transcriptomes for healthy and diseased stapes, and in combination with pathway analysis has helped identify genes and molecular processes dysregulated in otosclerotic tissue. Whole Exome Sequencing has been employed to investigate rare variants that segregate with otosclerosis in affected families, and has been followed by a variant filtering strategy, which has prioritised genes found to be dysregulated during RNA-sequencing. -

Mir-96 Regulates the Progression of Differentiation in Mammalian Cochlear Inner and Outer Hair Cells
miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells Stephanie Kuhna,1, Stuart L. Johnsona,1, David N. Furnessb, Jing Chenc, Neil Inghamc, Jennifer M. Hiltonc, Georg Steffesc, Morag A. Lewisc, Valeria Zampinia,d, Carole M. Hackneya, Sergio Masettod, Matthew C. Holleya, Karen P. Steelc, and Walter Marcottia,2 aDepartment of Biomedical Science, University of Sheffield, Sheffield S10 2TN, United Kingdom; bInstitute for Science and Technology in Medicine, Keele University, Keele ST5 5BG, United Kingdom; cWellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, United Kingdom; and dDepartment of Physiology, University of Pavia, 27100 Pavia, Italy Edited by Mary-Claire King, University of Washington, Seattle, WA, and approved December 27, 2010 (received for review November 8, 2010) MicroRNAs (miRNAs) are small noncoding RNAs able to regulate cells up to at least P5 (23) and in the spiral ganglion up to P14 a broad range of protein-coding genes involved in many biological (19). Mutations in miR-96 have been associated with non- processes. miR-96 is a sensory organ-specific miRNA expressed in syndromic progressive hearing loss in humans (15) and mice (23). the mammalian cochlea during development. Mutations in miR-96 We found that hair cell development in diminuendo mice with cause nonsyndromic progressive hearing loss in humans and mice. a Mir96 mutation is arrested at around the day of birth, a time The mouse mutant diminuendo has a single base change in the when IHCs and OHCs still exhibit qualitatively similar bio- seed region of the Mir96 gene leading to widespread changes in physical properties. -

Microrna-1182 and Let-7A Exert Synergistic Inhibition on Invasion
Pan et al. Cancer Cell Int (2021) 21:161 https://doi.org/10.1186/s12935-021-01797-z Cancer Cell International PRIMARY RESEARCH Open Access MicroRNA-1182 and let-7a exert synergistic inhibition on invasion, migration and autophagy of cholangiocarcinoma cells through down-regulation of NUAK1 Xin Pan*, Gang Wang and Baoming Wang Abstract Background: Cholangiocarcinoma (CCA) is the second most common primary liver malignancy worldwide. Several microRNAs (miRNAs) have been implicated as potential tumor suppressors in CCA. This study aims to explore the potential efects of miR-1182 and let-7a on CCA development. Methods: Bioinformatics analysis was conducted to screen diferentially expressed genes in CCA, Western blot analysis detected NUAK1 protein expression and RT-qPCR detected miR-1182, let-7a and NUAK1 expression in CCA tissues and cell lines. Dual luciferase reporter gene assay and RIP were applied to validate the relationship between miR-1182 and NUAK1 as well as between let-7a and NUAK1. Functional experiment was conducted to investigate the role of miR-1182, let-7a and NUAK1 in cell migration, proliferation and autophagy. Then, the CCA cells that received various treatments were implanted to mice to establish animal model, followed by tumor observation and HE staining to evaluate lung metastasis. Results: CCA tissues and cells were observed to have a high expression of NUAK1 and poor expression of miR-1182 and let-7a. NUAK1 was indicated as a target gene of miR-1182 and let-7a. Importantly, upregulation of either miR-1182 or let-7a induced autophagy, and inhibited cell progression and in vivo tumor growth and lung metastasis; moreover, combined treatment of miR-1182 and let-7a overexpression presented with enhanced inhibitory efect on NUAK1 expression and CCA progression, but such synergistic efect could be reversed by overexpression of NUAK1. -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Gene Section Review MIRN21 (microRNA 21) Sadan Duygu Selcuklu, Mustafa Cengiz Yakicier, Ayse Elif Erson Biology Department, Room: 141, Middle East Technical University, Ankara 06531, Turkey Published in Atlas Database: March 2007 Online updated version: http://AtlasGeneticsOncology.org/Genes/MIRN21ID44019ch17q23.html DOI: 10.4267/2042/38450 This work is licensed under a Creative Commons Attribution-Non-commercial-No Derivative Works 2.0 France Licence. © 2007 Atlas of Genetics and Cytogenetics in Oncology and Haematology sequences of MIRN21 showed enrichment for Pol II Identity but not Pol III. Hugo: MIRN21 MIRN21 gene was shown to harbor a 5' promoter Other names: hsa-mir-21; miR-21 element. 1008 bp DNA fragment for MIRN21 gene Location: 17q23.1 was cloned (-959 to +49 relative to T1 transcription Location base pair: MIRN21 is located on chr17: site, see Figure 1; A). Analysis of the sequence showed 55273409-55273480 (+). a candidate 'CCAAT' box transcription control element Local order: Based on Mapviewer, genes flanking located approximately about 200 nt upstream of the T1 MIRN21 oriented from centromere to telomere on site. T1 transcription site was found to be located in a 17q23 are: sequence similar to 'TATA' box - TMEM49, transmembrane protein 49, 17q23.1. (ATAAACCAAGGCTCTTACCATAGCTG). To test - MIRN21, microRNA 21, 17q23.1. the activity of the element, about 1kb DNA fragment - TUBD1, tubulin, delta 1, 17q23.1. was inserted into the 5' end of firefly luciferase - LOC729565, similar to NADH dehydrogenase indicator gene and transfected into 293T cells. The (ubiquinone) 1 beta subcomplex, 8, 19 kDa, 17q23.1. -

Identification of Mirnas and Their Targets Through High-Throughput
Chen et al. BMC Plant Biology (2016) 16:80 DOI 10.1186/s12870-016-0770-z RESEARCH ARTICLE Open Access Identification of miRNAs and their targets through high-throughput sequencing and degradome analysis in male and female Asparagus officinalis Jingli Chen1†, Yi Zheng2†, Li Qin1†, Yan Wang1, Lifei Chen1, Yanjun He1, Zhangjun Fei2,3 and Gang Lu1* Abstract Background: MicroRNAs (miRNAs), a class of non-coding small RNAs (sRNAs), regulate various biological processes. Although miRNAs have been identified and characterized in several plant species, miRNAs in Asparagus officinalis have not been reported. As a dioecious plant with homomorphic sex chromosomes, asparagus is regarded as an important model system for studying mechanisms of plant sex determination. Results: Two independent sRNA libraries from male and female asparagus plants were sequenced with Illumina sequencing, thereby generating 4.13 and 5.88 million final clean reads, respectively. Both libraries predominantly contained 24-nt sRNAs, followed by 21-nt sRNAs. Further analysis identified 154 conserved miRNAs, which belong to 26 families, and 39 novel miRNA candidates seemed to be specific to asparagus. Comparative profiling revealed that 63 miRNAs exhibited significant differential expression between male and female plants, which was confirmed by real-time quantitative PCR analysis. Among them, 37 miRNAs were significantly up-regulated in the female library, whereas the others were preferentially expressed in the male library. Furthermore, 40 target mRNAs representing 44 conserved and seven novel miRNAs were identified in asparagus through high-throughput degradome sequencing. Functional annotation showed that these target mRNAs were involved in a wide range of developmental and metabolic processes. -

WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/174282 A2 20 December 2012 (20.12.2012) P O P C T (51) International Patent Classification: David [US/US]; 13539 N . 95th Way, Scottsdale, AZ C12Q 1/68 (2006.01) 85260 (US). (21) International Application Number: (74) Agent: AKHAVAN, Ramin; Caris Science, Inc., 6655 N . PCT/US20 12/0425 19 Macarthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 14 June 2012 (14.06.2012) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, English (25) Filing Language: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, Publication Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/497,895 16 June 201 1 (16.06.201 1) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 61/499,138 20 June 201 1 (20.06.201 1) US OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, 61/501,680 27 June 201 1 (27.06.201 1) u s SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 61/506,019 8 July 201 1(08.07.201 1) u s TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Wo 2008/070082 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 12 June 2008 (12.06.2008) WO 2008/070082 A2 (51) International Patent Classification: (74) Agents: CLAUSS, Isabelle, M. et al.; Patent Group, FO A6IK 48/00 (2006.01) LEY HOAG LLP, 155 Seaport Blvd., Boston, MA 02210- 2600 (US). (21) International Application Number: PCT/US2007/024845 (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (22) International Filing Date: AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, 4 December 2007 (04.12.2007) CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, (25) Filing Language: English IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, (26) Publication Language: English MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, (30) Priority Data: PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, 60/872,764 4 December 2006 (04. 12.2006) US TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW (71) Applicants (for all designated States except US): THE JOHNS HOPKINS UNIVERSITY [US/US]; 3400 North (84) Designated States (unless otherwise indicated, for every Charles Street, Baltimore, MD 21218 (US). OHIO STATE kind of regional protection available): ARIPO (BW, GH, UNIVERSITY [US/US]; 1320 Kinnear Road, Columbus, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, OH 43212 (US). -

Mutations Suggests Both Loss-Of-Function and Gain-Of-Function Effects Morag A
© 2021. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2021) 14, dmm047225. doi:10.1242/dmm.047225 RESEARCH ARTICLE Hearing impairment due to Mir183/96/182 mutations suggests both loss-of-function and gain-of-function effects Morag A. Lewis1,2,*, Francesca Di Domenico1, Neil J. Ingham1,2, Haydn M. Prosser2 and Karen P. Steel1,2 ABSTRACT birth. However, this is not the cause of the hearing loss; even before The microRNA miR-96 is important for hearing, as point mutations in the onset of normal hearing, homozygote hair cells fail to mature humans and mice result in dominant progressive hearing loss. Mir96 both morphologically and physiologically, remaining in their is expressed in sensory cells along with Mir182 and Mir183, but the immature state, and heterozygote hair cells show a developmental roles of these closely-linked microRNAs are as yet unknown. Here, delay. miR-96 is thus thought to be responsible for coordinating we analyse mice carrying null alleles of Mir182, and of Mir183 and hair cell maturation (Chen et al., 2014; Kuhn et al., 2011). Mir96 together to investigate their roles in hearing. We found that Overexpression of the three miRNAs also results in cochlear defects Mir183/96 heterozygous mice had normal hearing and homozygotes and hearing loss (Weston et al., 2018). The complete loss of all were completely deaf with abnormal hair cell stereocilia bundles and mature miRNAs from the inner ear results in early developmental reduced numbers of inner hair cell synapses at 4 weeks of age. defects including a severely truncated cochlear duct (Friedman Mir182 knockout mice developed normal hearing then exhibited et al., 2009; Soukup et al., 2009). -

Timo Lassmann, Yoshiko Maida 2, Yasuhiro Tomaru, Mami Yasukawa
Int. J. Mol. Sci. 2015, 16, 1192-1208; doi:10.3390/ijms16011192 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Telomerase Reverse Transcriptase Regulates microRNAs Timo Lassmann 1,†, Yoshiko Maida 2,†, Yasuhiro Tomaru 1, Mami Yasukawa 2, Yoshinari Ando 1, Miki Kojima 1, Vivi Kasim 2, Christophe Simon 1, Carsten O. Daub 1, Piero Carninci 1, Yoshihide Hayashizaki 1,* and Kenkichi Masutomi 2,3,* 1 RIKEN Omics Science Center, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan; E-Mails: [email protected] (T.L.); [email protected] (Y.T.); [email protected] (Y.A.); [email protected] (M.K.); [email protected] (C.S.); [email protected] (C.O.D.); [email protected] (P.C.) 2 Cancer Stem Cell Project, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; E-Mails: [email protected] (Y.M.); [email protected] (M.Y.); [email protected] (V.K.) 3 Precursory Research for Embryonic Science and Technology (PREST), Japan Science and Technology Agency, 4-1-8 Honcho Kawaguchi, Saitama 332-0012, Japan † These authors contributed equally to this work. * Authors to whom correspondence should be addressed; E-Mails: [email protected] (Y.H.); [email protected] (K.M.); Tel.: +81-45-503-9248 (Y.H.); +81-3-3547-5173 (K.M.); Fax: +81-45-503-9216 (Y.H.); +81-3-3547-5123 (K.M.). Academic Editor: Martin Pichler Received: 22 November 2014 / Accepted: 26 December 2014 / Published: 6 January 2015 Abstract: MicroRNAs are small non-coding RNAs that inhibit the translation of target mRNAs. -
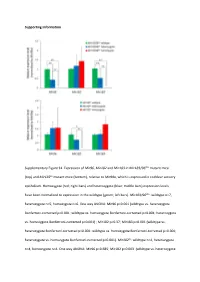
Supporting Information Supplementary Figure S1
Supporting Information Supplementary Figure S1. Expression of Mir96, Mir182 and Mir183 in Mir183/96dko mutant mice (top) and Mir182ko mutant mice (bottom), relative to Mir99a, which is expressed in cochlear sensory epithelium. Homozygote (red; right bars) and heterozygote (blue; middle bars) expression levels have been normalised to expression in the wildtype (green; left bars). Mir183/96dko: wildtype n=7, heterozygote n=5, homozygote n=6. One way ANOVA: Mir96 p<0.001 (wildtype vs. heterozygote Bonferroni‐corrected p<0.001; wildtype vs. homozygote Bonferroni‐corrected p<0.001; heterozygote vs. homozygote Bonferroni‐corrected p=0.001) ; Mir182 p=0.37; Mir183 p<0.001 (wildtype vs. heterozygote Bonferroni‐corrected p=0.001; wildtype vs. homozygote Bonferroni‐corrected p<0.001; heterozygote vs. homozygote Bonferroni‐corrected p<0.001). Mir182ko: wildtype n=4, heterozygote n=4, homozygote n=4. One way ANOVA: Mir96 p=0.685; Mir182 p=0.003 (wildtype vs. heterozygote Bonferroni‐corrected p=0.397; wildtype vs. homozygote Bonferroni‐corrected p=0.003; heterozygote vs. homozygote Bonferroni‐corrected p=0.032); Mir183 p=0.04 (wildtype vs. heterozygote Bonferroni‐corrected p=1.0; wildtype vs. homozygote Bonferroni‐corrected p=0.068; heterozygote vs. homozygote Bonferroni‐corrected p=0.094), Error bars are standard deviation (* = P < 0.05, ** = P ≤ 0.01). Supplementary Figure S2. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir183/96dko mice at all ages tested. Number of mice of each genotype tested at each age is shown on the threshold plot. Supplementary Figure S3. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir182ko mice at all ages tested. -

The Role of Micrornas in Oral Squamous Cell Carcinoma Pathogenesis: a Literature Review
Applied Cancer Research. 2013;33(4):198-205. REVIEW The role of microRNAS in oral squamous cell carcinoma pathogenesis: a literature review Anne Maria Guimarães Lessa1, Ludmila Faro Valverde2, Rosane Borges Dias2, Maria Cecília Mathias Machado1, Jean Nunes dos Santos3, Clarissa Araújo Gurgel Rocha3 ABSTRACT In this review, we summarize the main aspects related to the involvement of microRNAs (miRNAs) in oral carcinogenesis. miRNAs are small non-protein-coding RNAs that function such as regulators of gene expression. They regulate various biological processes such as growth, differentiation and apoptosis and have been widely studied in carcinogenesis. miRNAs may exhibit oncogenic or tumor suppressor activity in cancer, depending on the biological context and the cell type. The altered expression patterns of miRNA in cancer could serve as molecular biomarkers for tumor diagnosis, prognosis and disease-specific prediction of therapeutic responses. The literature indicates that up-regulation of miR-21, miR-221, miR-184 and under expression of miR-133a, miR-375 and let-7b are the principal profile in oral squamous cell carcinomas. Keywords: gene expression; microRNAS; MIRN133 microRNA, human; MIRN184 microRNA, human; MIRN21 microRNA, human; MIRN221 microRNA, human; MIRN375 microRNA, human; mirnlet7 microRNA, human; mouth mucosa; mouth neoplasms; tumor markers, biological. INTRODUCTION years later, Reinhart et al.12 observed that another C. elegans heterochronic gene, let-7, was also represented by a small Cells have developed several biological mechanisms to non-coding RNA capable of starting the temporal cascade of ensure that mitosis, differentiation and death occur in a coor- regulatory genes through an RNA-RNA interaction with the 3 dinated manner and disturbances in genes related with these untranslated region (UTR) of target genes. -

Full-Text (PDF)
Original Article Iran J Ped Hematol Oncol. 2019, Vol9, No4, 49-56 Effect of Estradiol on miR-21& miR-155 Expression in promyelocytic leukemia-derived cell line NB4 Robab Naghibzadeh MSc1, Narges Obeidi PhD1,2,*, Alireza Farsinejd PhD3,Gholamreza Khamisipour PhD1, Masoud TohidFar PhD4 1. Department of Hematology, School of Para Medicine, Bushehr University of Medical Sciences, Bushehr, Iran 2. Blood Transfusion Organization, Bushehr, Iran 3. Department of Hematology and Medical Laboratory Sciences, Faculty of Allied Medical Sciences, Kerman University of Medical Sciences, Kerman, Iran 4. Department of Hematology and Medical Laboratory Sciences, Faculty of Allied Medical Sciences, ShahidBeheshti University of Medical Sciences, Tehran, Iran *Corresponding author:Dr Narges Obeidi, Department of Hematology, School of Para Medicine, Bushehr University of Medical Sciences, Bushehr, Iran. E-mail: [email protected]. Orchid ID:0000-0001-8087-2850 Received: 25 March 2019 Accepted: 10 November 2019 Abstract Background: Due to the estrogen participation in modulating the proliferation and commitment of stem cells and the effects of miR-21 and miR-155 expression on reduced proliferation and colony formation of acute myeloid leukemia (AML), the aim of the present study was to evaluate the effect of estradiol on expression of miR-21 and miR-155 in the NB4 cell line, as an acute promyelocytic leukemia (APL). Materials and Methods: In the present experiment, NB4 cells were treated with different quantities of estradiol (5, 25, 50, 75, 100, 150, 200, 250 μg/ml) and vehicle control for 24 and 48 hours. Viability, apoptosis, and cellular proliferation were estimated by trypan blue exclusion, flow cytometry, and MTT assays, respectively.