Territoriality and Nest Competition in the Two-Spotted Goby (Pomatoschistus Flavescens)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reef Fishes of the Bird's Head Peninsula, West
Check List 5(3): 587–628, 2009. ISSN: 1809-127X LISTS OF SPECIES Reef fishes of the Bird’s Head Peninsula, West Papua, Indonesia Gerald R. Allen 1 Mark V. Erdmann 2 1 Department of Aquatic Zoology, Western Australian Museum. Locked Bag 49, Welshpool DC, Perth, Western Australia 6986. E-mail: [email protected] 2 Conservation International Indonesia Marine Program. Jl. Dr. Muwardi No. 17, Renon, Denpasar 80235 Indonesia. Abstract A checklist of shallow (to 60 m depth) reef fishes is provided for the Bird’s Head Peninsula region of West Papua, Indonesia. The area, which occupies the extreme western end of New Guinea, contains the world’s most diverse assemblage of coral reef fishes. The current checklist, which includes both historical records and recent survey results, includes 1,511 species in 451 genera and 111 families. Respective species totals for the three main coral reef areas – Raja Ampat Islands, Fakfak-Kaimana coast, and Cenderawasih Bay – are 1320, 995, and 877. In addition to its extraordinary species diversity, the region exhibits a remarkable level of endemism considering its relatively small area. A total of 26 species in 14 families are currently considered to be confined to the region. Introduction and finally a complex geologic past highlighted The region consisting of eastern Indonesia, East by shifting island arcs, oceanic plate collisions, Timor, Sabah, Philippines, Papua New Guinea, and widely fluctuating sea levels (Polhemus and the Solomon Islands is the global centre of 2007). reef fish diversity (Allen 2008). Approximately 2,460 species or 60 percent of the entire reef fish The Bird’s Head Peninsula and surrounding fauna of the Indo-West Pacific inhabits this waters has attracted the attention of naturalists and region, which is commonly referred to as the scientists ever since it was first visited by Coral Triangle (CT). -

Taxonomic Research of the Gobioid Fishes (Perciformes: Gobioidei) in China
KOREAN JOURNAL OF ICHTHYOLOGY, Vol. 21 Supplement, 63-72, July 2009 Received : April 17, 2009 ISSN: 1225-8598 Revised : June 15, 2009 Accepted : July 13, 2009 Taxonomic Research of the Gobioid Fishes (Perciformes: Gobioidei) in China By Han-Lin Wu, Jun-Sheng Zhong1,* and I-Shiung Chen2 Ichthyological Laboratory, Shanghai Ocean University, 999 Hucheng Ring Rd., 201306 Shanghai, China 1Ichthyological Laboratory, Shanghai Ocean University, 999 Hucheng Ring Rd., 201306 Shanghai, China 2Institute of Marine Biology, National Taiwan Ocean University, Keelung 202, Taiwan ABSTRACT The taxonomic research based on extensive investigations and specimen collections throughout all varieties of freshwater and marine habitats of Chinese waters, including mainland China, Hong Kong and Taiwan, which involved accounting the vast number of collected specimens, data and literature (both within and outside China) were carried out over the last 40 years. There are totally 361 recorded species of gobioid fishes belonging to 113 genera, 5 subfamilies, and 9 families. This gobioid fauna of China comprises 16.2% of 2211 known living gobioid species of the world. This report repre- sents a summary of previous researches on the suborder Gobioidei. A recently diagnosed subfamily, Polyspondylogobiinae, were assigned from the type genus and type species: Polyspondylogobius sinen- sis Kimura & Wu, 1994 which collected around the Pearl River Delta with high extremity of vertebral count up to 52-54. The undated comprehensive checklist of gobioid fishes in China will be provided in this paper. Key words : Gobioid fish, fish taxonomy, species checklist, China, Hong Kong, Taiwan INTRODUCTION benthic perciforms: gobioid fishes to evolve and active- ly radiate. The fishes of suborder Gobioidei belong to the largest The gobioid fishes in China have long received little group of those in present living Perciformes. -

Tending Burrows of Monogamous Goby, Valenciennea Longipinnis (Lay Et Bennett)
Journal of Experimental Marine Biology and Ecology 253 (2000) 149±163 www.elsevier.nl/locate/jembe The role of mounds in promoting water-exchange in the egg- tending burrows of monogamous goby, Valenciennea longipinnis (Lay et Bennett) Takeshi Takegaki* , Akinobu Nakazono Department of Fisheries, Faculty of Agriculture, Kyushu University,6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan Received 22 December 1999; received in revised form 19 June 2000; accepted 29 June 2000 Abstract Valenciennea longipinnis spawns monogamously in a burrow. After spawning, the paired female constructs a conspicuous mound on the burrow by carrying and piling up substratum-derived materials while the male tends eggs in the burrow until hatching occurs. In this study, the mounds of V. longipinnis were tested in the ®eld to con®rm their function of promoting water-exchange in the burrow, and their ecological role was examined in relation to egg care by the male. The mound of V.longipinnis promoted water-exchange in the burrow, contributing to the provision of external oxygenated sea water into the burrow. Therefore, dissolved oxygen (DO) concentrations in the burrow with a mound were signi®cantly higher than those without a mound. Although male egg-tending behavior (e.g., fanning) may also promote water-exchange in the burrow, the water-exchange appeared to depend mainly on the hydrodynamic effect. Removals of the mound and paired female on the day of spawning led to high rates of egg-desertion by males. Since the frequency and time of fanning increase with a decrease of DO concentration in the burrow, the egg-desertion may result from an increased parental cost to males due to the decrease of water-exchange without a mound. -

Complete Sequence of the Mitochondrial Genome of Odontamblyopus Rubicundus (Perciformes: Gobiidae): Genome Characterization and Phylogenetic Analysis
c Indian Academy of Sciences RESEARCH ARTICLE Complete sequence of the mitochondrial genome of Odontamblyopus rubicundus (Perciformes: Gobiidae): genome characterization and phylogenetic analysis TIANXING LIU, XIAOXIAO JIN, RIXIN WANG∗ and TIANJUN XU∗ Laboratory for Marine Living Resources and Molecular Engineering, College of Marine Science, Zhejiang Ocean University, Zhoushan 316000, People’s Republic of China Abstract Odontamblyopus rubicundus is a species of gobiid fishes, inhabits muddy-bottomed coastal waters. In this paper, the first complete mitochondrial genome sequence of O. rubicundus is reported. The complete mitochondrial genome sequence is 17119 bp in length and contains 13 protein-coding genes, two rRNA genes, 22 tRNA genes, a control region and an L-strand origin as in other teleosts. Most mitochondrial genes are encoded on H-strand except for ND6 and seven tRNA genes. Some overlaps occur in protein-coding genes and tRNAs ranging from 1 to 7 bp. The possibly nonfunctional L-strand origin folded into a typical stem-loop secondary structure and a conserved motif (5-GCCGG-3) was found at the base of the stem within the tRNACys gene. The TAS, CSB-2 and CSB-3 could be detected in the control region. However, in contrast to most of other fishes, the central conserved sequence block domain and the CSB-1 could not be recognized in O. rubicundus,whichis consistent with Acanthogobius hasta (Gobiidae). In addition, phylogenetic analyses based on different sequences of species of Gobiidae and different methods showed that the classification of O. rubicundus into Odontamblyopus due to morphology is debatable. [Liu T., Jin X., Wang R. and Xu T. -

Habitat Structure, Disturbance and the Composition of Sand-Dwelling Goby Assemblages in a Coral Reef Lagoon
MARINE ECOLOGY PROGRESS SERIES Vol. 268: 221–230, 2004 Published March 9 Mar Ecol Prog Ser Habitat structure, disturbance and the composition of sand-dwelling goby assemblages in a coral reef lagoon Craig Syms1, 2,*, Geoffrey P. Jones1 1School of Marine Biology and Aquaculture, James Cook University, Townsville, Queensland 4811, Australia 2Present address: Long Marine Laboratory and Department of Ecology and Evolutionary Biology, University of California, 100 Shaffer Road, Santa Cruz, California 95060, USA ABSTRACT: Coral reef lagoons and back reef areas are composed more of sand than hard reef habi- tat. They support a diverse mix of fishes, including species restricted to sandy habitats and those dependent on both hard and soft substrata. However the resident assemblages associated with sand and the factors affecting their distribution and abundance are poorly understood. Here we examine spatial co-variation in the abundance of burrowing goby assemblages and habitat characteristics in the lagoon at Lizard Island (Great Barrier Reef). The aim was to identify which key habitat-variables should be incorporated into models to predict the structure of sand-dwelling fish communities. We focused on 10 common sand goby species from 7 genera: Amblyeleotris, Cryptocentrus, Ctenogob- iops and Vanderhorstia (associated with burrows constructed by alpheid shrimps), and Amblygobius, Oplopomus and Valenciennea (free-living, burrowing species). Spatial patterns were examined by stratifying the lagoon into 6 recognizable habitat zones, and conducting visual transects in replicate sites within each zone. The abundance of all goby species encountered and habitat variables (depth, distance from reef, topography, disturbance of different types, sediment composition) were recorded in each transect. -
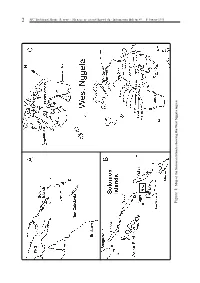
An Analysis of the West Nggela (Solomon Islands) Fish Taxonomy
2 SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 Map of the Solomon Islands showing West Nggela region Figure 1: Figure SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 3 What’s in a name? An analysis of the West Nggela (Solomon Islands) fish taxonomy. by Simon Foale 1 Introduction Lobotidae, Gerreidae, Sparidae, Ephippidae, Chaetodontidae, Pomacentridae, Cirhitidae, Accurate knowledge about the behaviour, biol- Polynemidae, Labridae, Opistognathidae, ogy and ecology of organisms comprising marine Trichonotidae, Pinguipedidae, Blenniidae, fisheries is a vital prerequisite for their manage- Gobiidae, Microdesmidae, Zanclidae, Bothidae, ment. Before beginning any study on local knowl- Pleuronectidae, and Soleidae. edge of marine fauna, a working knowledge of The English names of many species of fish vary their local names must be obtained. Moreover, a quite a bit, even within one country such as great deal of local knowledge can often emerge in Australia. For most of the species listed in the very process of obtaining names (Ruddle, Appendix 1, I have used the English names given 1994). A detailed treatment of the local naming by Randall et al. (1990). For species not included in system of West Nggela marine fauna is given in Randall et al. (1990), names from Kailola (1987a, b, this paper. 1991) were used. Methods Results Local names of fish were collected by asking Appendix 1 contains 350 unique Nggela folk people to provide the Nggela names for fishes taxa for cartilaginous and bony fishes, together from photographs in books featuring most of the with the scientific (Linnean) taxa they correspond common Indo-Pacific species (Randall et al., 1990 to and, where available, a brief note describing an and Myers, 1991). -

André Phillips Phd Thesis
THE MECHANISMS AND CONSEQUENCES OF OVIPOSITION DECISIONS IN THE EUROPEAN BITTERLING André Phillips A Thesis Submitted for the Degree of PhD at the University of St Andrews 2018 Full metadata for this item is available in St Andrews Research Repository at: http://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/15524 This item is protected by original copyright The mechanisms and consequences of oviposition decisions in the European bitterling André Phillips 1. Candidate’s declarations: I, André Phillips hereby certify that this thesis, which is approximately 38,000 words in length, has been written by me, and that it is the record of work carried out by me, or principally by myself in collaboration with others as acknowledged, and that it has not been submitted in any previous application for a higher degree. I was admitted as a research student in October, 2014 and as a candidate for the degree of PhD in October, 2014; the higher study for which this is a record was carried out in the University of St Andrews between 2014 and 2017. I, André Phillips, received assistance in the writing of this thesis in respect of grammar & spelling, which was provided by Helen Spence-Jones Date …… signature of candidate ……… 2. Supervisor’s declaration: I hereby certify that the candidate has fulfilled the conditions of the Resolution and Regulations appropriate for the degree of ……… in the University of St Andrews and that the candidate is qualified to submit this thesis in application for that degree. -

Checklist of the Coral Fish Fauna of Xisha Islands, China
Biodiversity Data Journal 9: e63945 doi: 10.3897/BDJ.9.e63945 Taxonomic Paper Checklist of the coral fish fauna of Xisha Islands, China Shuting Qiu‡, Bin Chen ‡,§, Jianguo Du‡,§, Kar-Hoe Loh |, Jianji Liao‡¶, Xinming Liu , Wen Yang‡ ‡ Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China § Fujian Provincial Key Laboratory ofMarine Ecological Conservation and Restoration, Xiamen, China | Institute of Ocean and Earth Sciences, University of Malaya, Kuala Lumpur, Malaysia ¶ Guangxi University of Chinese Medicine, Guangxi, China Corresponding author: Jianguo Du ([email protected]) Academic editor: Yahui Zhao Received: 04 Feb 2021 | Accepted: 01 Mar 2021 | Published: 08 Mar 2021 Citation: Qiu S, Chen B, Du J, Loh K-H, Liao J, Liu X, Yang W (2021) Checklist of the coral fish fauna of Xisha Islands, China. Biodiversity Data Journal 9: e63945. https://doi.org/10.3897/BDJ.9.e63945 Abstract Background The Xisha Islands are composed of the Yongle Islands and the Xuande Islands in Hainan Province, China. It has one of the highest species diversity in the world and is also a typical oceanic distribution area of coral reefs globally. The ichthyofauna of the Xisha Islands were recorded by underwater visual census in May 2019 and July 2020. The survey data were combined with previous records of species into the checklist of the Xisha Islands presented herein. A total of 691 species, belonging to 24 orders and 97 families, was recorded. The major families were Labridae, Pomacentridae, Serranidae, Chaetodontidae, Hexanchidae, Lutjanidae, Scaridae, Gobiidae, Scorpaenidae and Carangidae. In this study, the Coral Fish iversity Index (CFDI) of six families (Chaetodontidae, Pomacanthidae, Pomacentridae, Labridae, Scaridae and Acanthuridae) was 229, indicating 756 coral fishes. -

View/Download
GOBIIFORMES (part 7) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 15.0 - 24 May 2021 Order GOBIIFORMES (part 7 of 7) Family GOBIIDAE Gobies (Rhinogobiops through Zebrus) Taxonomic note: includes taxa formerly included in the families Kraemeriidae, Microdesmidae and Schindleriidae. Rhinogobiops Hubbs 1926 -ops, appearance, i.e., similar to Rhinogobius (Oxudercidae) Rhinogobiops nicholsii (Bean 1882) in honor of Capt. Henry E. Nichols (d. 1899), U.S. Navy, commander of the United States Coast and Geogetic Survey steamer Hassler through the inland waters of British Columbia and southern Alaska; he “secured” type and preserved 31 species in total, all in a state of “excellent preservation”; in fact, Bean wrote, “It is due to Captain Nichols to say that no better-preserved lot of fishes has been received from any other collector.” Risor Ginsburg 1933 one who laughs, allusion not explained, perhaps referring to tusk-like teeth protruding below flaring upper lip of Garmannia binghami (=R. ruber) Risor ruber (Rosén 1911) red, referring to its brownish-red coloration Robinsichthys Birdsong 1988 in honor of C. Richard Robins (1928-2020), for his many contributions to our knowledge of American gobies; ichthys, fish Robinsichthys arrowsmithensis Birdsong 1988 -ensis, suffix denoting place: Arrowsmith Bank, Caribbean Sea, type locality Schindleria Giltay 1934 -ia, belonging to: German zoologist Otto Schindler (1906-1959), who described the first two species in the genus Schindleria brevipinguis Watson & Walker 2004 brevis, short, referring to its size, reaching 8.4 mm SL, believed at the time to be the world’s smallest vertebrate; pinguis, stout, referring to deeper, broader body compared to congeners Schindleria elongata Fricke & Abu El-Regal 2017 elongate, referring to more slender body compared to the closely related S. -

A List of Fishes Inhabiting Mangroves from the South-West Lagoon Of
Micronesica 29(1): 1- 19, 1996 A List of Fishes Inhabiting Mangroves from the South-West Lagoon of New Caledonia PIERRE THOLLOT Consultant en Environnement et Ressources Marines BP 9239-98 807 Noumea Cedex, New Caledonia Abstract-Mangrove fishes from the South-West Lagoon of New Cal edonia were sampled using gill nets, fyke nets and rotenone poisonings from 1987 to 1990. A total of 262 species, distributed among 64 families, were recorded with 3 new records. The most speciose families are Go biidae (31 spp.), Apogonidae (17 spp.), Serranidae (13 spp.), Carangidae ( 11 spp. ),M uraenidae and Lethrinidae ( 10 spp. ). Estuarine families, such as Elopidae, Kuhliidae, Cichlidae, Chandidae and Microdesmidae, are also present together with reef or soft bottom associated species (includ ing Serranidae, Lutjanidae, Carangidae and Lethrinidae). Sciaenidae and Ariidae were not censused and freshwater species were scarce. Introduction Mangroves are tropical tidal forests and swamps. In spite of the benefits they provide such as land protection, sediment trapping, high primary productivity, enhancement of coastal productivity and fishery yields, mangroves are severely threatened on most shorelines (Chapman 1976, Baines 1981, Christensen 1983, Saenger et al. 1983, Lal 1984, Tomlinson 1986, Thollot 1992a, in press). Extensive losses of mangrove forests have sometimes reached dramatic levels of disturbance, up to 60% of total area in Java (Saenger et al. 1983). These are mainly related to land reclamation and conversion, particularly for agriculture (rice or sugar cane farming), aquaculture (prawns and fish ponds) or tourism (artificial beaches, re sorts). There is an urgent need to prevent further destruction of mangroves, to allow a sustainable use of this natural resource (Thollot, in press). -
Functional Niche Partitioning in Herbivorous Coral Reef Fishes
ResearchOnline@JCU This file is part of the following reference: Brandl, Simon Johannes (2016) Functional niche partitioning in herbivorous coral reef fishes. PhD thesis, James Cook University. Access to this file is available from: http://researchonline.jcu.edu.au/45253/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://researchonline.jcu.edu.au/45253/ Functional niche partitioning in herbivorous coral reef fishes Thesis submitted by: Simon Johannes Brandl January 2016 For the degree: Doctor of Philosophy College of Marine and Environmental Sciences ARC Centre of Excellence for Coral Reef Studies James Cook University i Acknowledgements I am deeply indebted to my supervisor, David Bellwood, whose invaluable intellectual and emotional support has been the cornerstone of my degree. His outstanding guidance, astute feedback, incredible generosity, and tremendous patience cannot be credited adequately within the scope of this acknowledgements section. Besides his supervisory contribution to my degree, I am grateful for the countless hours full of cheerful negotiations, curly remarks, philosophical debates, humorous chitchat, and priceless counselling. I also thank everybody who has helped me in the field: Jordan Casey, Christopher Goatley, Jennifer Hodge, James Kerry, Michael Kramer, Katia Nicolet, Justin Welsh, and the entire staff of Lizard Island Research Station. I am especially grateful for Christopher Mirbach’s help, commitment, and loyalty throughout many weeks of fieldwork. This thesis would have been impossible without his dedication and enthusiasm for marine fieldwork. -

Habitat, Growth and Life History of the Goby Parapocryptes Serperaster
Habitat, growth and life history of the goby Parapocryptes serperaster Minh Quang Dinh, BSc. MSc. A thesis submitted for the degree of Doctor of Philosophy School of Biological Sciences Faculty of Science & Engineering Flinders University December 2015 CONTENTS CONTENTS.............................................................................................................. i LIST OF TABLES ................................................................................................... v LIST OF FIGURES ................................................................................................. vi DECLARATION .................................................................................................. viii ABSTRACT............................................................................................................ ix ACKNOWLEDGEMENT ....................................................................................... xi CHAPTER 1: GENERAL INTRODUCTION .......................................................... 1 1.1. Coastal and estuarine ecosystems and fisheries .............................................. 1 1.2. Coastal and estuarine fisheries in the Mekong Delta....................................... 2 1.3. Habitat use and features ................................................................................. 6 1.4. Growth pattern and condition factor variation ................................................ 7 1.5. Food and feeding habit .................................................................................