Assessment of the Implications of Interactions Between Fur Seals And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cabinet Cover Sheet Title Protection of the White
CABINET COVER SHEET TITLE PROTECTION OF THE WHITE SHARK- REGULATIONS UNDER THE FISHERIES ACT 1982 AND THE NATIONAL PARKS AND WILDLIFE ACT 1972 MINISTERS HON DAVID WOTTON MP MINISTER FOR THE ENVIRONMENT AND NATURAL RESOURCES. HON ROB KERIN MP MINISTER FOR PRIMARY INDUSTRIES. PURPOSE To provide for the total protection of the white shark and to effect the management of the cage viewing charter industry. RESOURCES REQUIRED No cost implications to government FOR IMPLEMENTATION No effect on government staffing. RELATIONSHIP TO The proposal is consistent with government GOVERNMENT POLICY policy that the State's fisheries resources and habitat are managed on a sustainable basis. CONSULTATION Public consultation took place with the issue of a discussion paper and releases in the media. FAMILY IMPACT STATEMENT Not applicable. 8. URGENCY Not applicable. 9. RECOMMENDATIONS It is recommended that Cabinet approve - 4.1 The drafting of regulations to amend the Fisheries (General) Regulations 1984 as follows - 4.1.1 to proclaim the white shark {Carcharodon carcharias) a protected species under regulation 6, prohibiting the capture, holding or killing of this species; 4.1.2 to amend regulation 35C to: (a) prohibit the use of blood, bone, meat, offal or skin of an animal (other than in a rock lobster pot or fish trap) within two nautical miles of the mainland of the State or all islands and reefs of the State which are exposed at low water mark; and (b) prohibit the depositing of or use of a mammal or any product of a mammal in all marine waters of South Australia; 4.1.3 to prohibit the use of wire trace with a gauge of 2mm or greater, in conjunction with fishing hooks greater than size 12^/0, in all waters of South Australia. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

Special Issue3.7 MB
Volume Eleven Conservation Science 2016 Western Australia Review and synthesis of knowledge of insular ecology, with emphasis on the islands of Western Australia IAN ABBOTT and ALLAN WILLS i TABLE OF CONTENTS Page ABSTRACT 1 INTRODUCTION 2 METHODS 17 Data sources 17 Personal knowledge 17 Assumptions 17 Nomenclatural conventions 17 PRELIMINARY 18 Concepts and definitions 18 Island nomenclature 18 Scope 20 INSULAR FEATURES AND THE ISLAND SYNDROME 20 Physical description 20 Biological description 23 Reduced species richness 23 Occurrence of endemic species or subspecies 23 Occurrence of unique ecosystems 27 Species characteristic of WA islands 27 Hyperabundance 30 Habitat changes 31 Behavioural changes 32 Morphological changes 33 Changes in niches 35 Genetic changes 35 CONCEPTUAL FRAMEWORK 36 Degree of exposure to wave action and salt spray 36 Normal exposure 36 Extreme exposure and tidal surge 40 Substrate 41 Topographic variation 42 Maximum elevation 43 Climate 44 Number and extent of vegetation and other types of habitat present 45 Degree of isolation from the nearest source area 49 History: Time since separation (or formation) 52 Planar area 54 Presence of breeding seals, seabirds, and turtles 59 Presence of Indigenous people 60 Activities of Europeans 63 Sampling completeness and comparability 81 Ecological interactions 83 Coups de foudres 94 LINKAGES BETWEEN THE 15 FACTORS 94 ii THE TRANSITION FROM MAINLAND TO ISLAND: KNOWNS; KNOWN UNKNOWNS; AND UNKNOWN UNKNOWNS 96 SPECIES TURNOVER 99 Landbird species 100 Seabird species 108 Waterbird -

Great Australian Bight BP Oil Drilling Project
Submission to Senate Inquiry: Great Australian Bight BP Oil Drilling Project: Potential Impacts on Matters of National Environmental Significance within Modelled Oil Spill Impact Areas (Summer and Winter 2A Model Scenarios) Prepared by Dr David Ellis (BSc Hons PhD; Ecologist, Environmental Consultant and Founder at Stepping Stones Ecological Services) March 27, 2016 Table of Contents Table of Contents ..................................................................................................... 2 Executive Summary ................................................................................................ 4 Summer Oil Spill Scenario Key Findings ................................................................. 5 Winter Oil Spill Scenario Key Findings ................................................................... 7 Threatened Species Conservation Status Summary ........................................... 8 International Migratory Bird Agreements ............................................................. 8 Introduction ............................................................................................................ 11 Methods .................................................................................................................... 12 Protected Matters Search Tool Database Search and Criteria for Oil-Spill Model Selection ............................................................................................................. 12 Criteria for Inclusion/Exclusion of Threatened, Migratory and Marine -

Ceduna 3D Marine Seismic Survey, Great Australian Bight
Referral of proposed action Project title: Ceduna 3D Marine Seismic Survey, Great Australian Bight 1 Summary of proposed action 1.1 Short description BP Exploration (Alpha) Limited (BP) proposes to undertake the Ceduna three-dimensional (3D) marine seismic survey across petroleum exploration permits EPP 37, EPP 38, EPP 39 and EPP 40 located in the Great Australian Bight (GAB). The proposed survey area is located in Commonwealth marine waters of the Ceduna sub-basin, between 1000 m and 3000 m deep, and is about 400 km west of Port Lincoln and 300 km southwest of Ceduna in South Australia. The proposed seismic survey is scheduled to commence no earlier than October 2011 and to conclude no later than end of May 2012. The survey is expected to take approximately six months to complete allowing for typical weather downtime. Outside this time window, metocean conditions become unsuitable for 3D seismic operations. The survey will be conducted by a specialist seismic survey vessel towing a dual seismic source array and 12 streamers, each 8,100 m long. 1.2 Latitude and longitude The proposed survey area is shown in Figure 1 with boundary coordinates provided in Table 1. Table 1. Boundary coordinates for the proposed survey area (GDA94) Point Latitude Longitude 1 35°22'15.815"S 130°48'50.107"E 2 35°11'50.810"S 131°02'16.061"E 3 35°02'37.061"S 131°02'15.972"E 4 35°24'55.520"S 131°30'41.981"E 5 35°14'38.653"S 131°42'16.982"E 6 35°00'47.460"S 131°41'40.052"E 7 34°30'09.196"S 131°02'44.991"E 8 34°06'27.572"S 131°02'11.557"E 9 33°41'24.007"S 130°31'04.931"E 10 33°41'25.575"S 130°15'22.936"E 11 34°08'47.552"S 130°12'34.972"E 12 34°09'16.169"S 129°41'03.591"E 13 34°18'22.970"S 129°29'32.951"E BP Ceduna 3D MSS Referral Page 1 of 48 1.3 Locality and property description The proposed seismic survey will take place in the permit areas for EPP 37, EPP 38, EPP 39 and EPP 40. -

Wilderness Protection Act 1992
Wilderness Protection Act 1992 ANNUAL REPORT 1 July 2014 to 30 June 2015 Wilderness Protection Act 1992 Annual Report 2014-15 For further information please contact: Manager, Protected Areas Department of Environment, Water and Natural Resources GPO Box 1047 ADELAIDE SA 5001 Telephone: (08) 8124 4707 Website: www.environment.sa.gov.au ABN: 36 702 093 234 ISSN: 1832-9357 IBSN: 978-1-921800-72-6 September 2015 TABLE OF CONTENTS LETTER OF TRANSMITTAL ................................................................................... 4 WILDERNESS PROTECTION ACT 1992 ANNUAL REPORT ..................................... 5 OBJECTS OF THE WILDERNESS PROTECTION ACT ............................. 5 ADMINISTRATION OF THE ACT ...................................................... 5 IDENTIFICATION OF LAND UNDER THE ACT ..................................... 5 CONSTITUTION OF LAND UNDER THE ACT ....................................... 6 ANNUAL REPORTING REQUIREMENTS ............................................ 6 NAMES, LOCATIONS AND QUALITY OF WILDERNESS PROTECTION AREAS AND ZONES ................................................................................ 7 EXTENT OF MINING OPERATIONS IN WILDERNESS PROTECTION ZONES14 MANAGEMENT OF WILDERNESS PROTECTION AREAS AND ZONES ...... 14 MANAGEMENT PLANS ADOPTED ................................................... 16 DECLARATION OF PROHIBITED AREAS .......................................... 17 EXPENDITURE ON MANAGEMENT OF WILDERNESS PROTECTION AREAS17 MONIES RECEIVED AND EXPENDED .............................................. -

Species Threatenedaustralian Sea-Lion Neophoca Cinerea
Australian Species ThreatenedAustralian Sea-lion Neophoca cinerea Conservation Status What does it look like? of colony sites is shallow, protected pools in which pups congregate. The waters The Australian Sea-lion is a handsome adjacent to breeding colonies are also pinniped—fin-footed mammal—with a important feeding areas. blunt snout and tightly rolled external ears Unlike other pinnipeds that were with front and hind flippers. Pinnipeds are harvested in Australia during the late 18th, marine mammals, which includes seals, 19th and early 20th centuries, Australian sea-lions and walruses. Sea-lion populations have not yet recovered, Australian Sea-lion males are typically and at some localities there is recent chocolate brown and can reach more evidence of continued population decline. than 2 metres in length and weigh up to Australian Sea-lion Point Labatt. The estimated size of the Australian Sea- Photo by WWF-Canon/John Gibbons 300 kilograms. Females are smaller and lion population is less than 10,000, with their colouring is generally silvery ash-grey 80 per cent occurring in South Australia Commonwealth: Vulnerable above and yellow to cream on their under- and 20 per cent in Western Australia. (Environment Protection and parts. Females can grow to more than Only five of the 73 known breeding sites 1.5 metres in length and weigh up to Biodiversity Conservation for Australian Sea-lions produce more than 80 kilograms. Act 1999) 100 pups each year, representing 57 per cent of all pups born. These sites, all Where is it found? located in South Australia, are Dangerous South Australia: Rare Reef, The Pages Islands, West Waldegrave The Australian Sea-lion is the only (National Parks and Wildlife Island, Seal Bay and Olive Island. -

Influence of Environmental Factors on Population Structure of Arrow Squid Nototodarus Gouldi: Implications for Stock Assessment
INFLUENCE OF ENVIRONMENTAL FACTORS ON POPULATION STRUCTURE OF ARROW SQUID NOTOTODARUS GOULDI: IMPLICATIONS FOR STOCK ASSESSMENT COREY PAUL GREEN, BAPPSC (FISHERIES) SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF TASMANIA OCTOBER 2011 Arrow squid Nototodarus gouldi (McCoy, 1888) (Courtesy of Robert Ingpen, 1974) FRONTISPIECE DECLARATION STATEMENT OF ORIGINALITY This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of the my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. ………………………………………….…. 28th October 2011 Corey Paul Green Date AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968. ………………………………………….…. 28th October 2011 Corey Paul Green Date I ACKNOWLEDGEMENTS This thesis assisted in fulfilling the objectives of the Fisheries Research and Development Corporation Project No. 2006/012 ―Arrow squid — stock variability, fishing techniques, trophic linkages — facing the challenges‖. Without such assistance this thesis would not have come to fruition. Research on statolith element composition was kindly funded by the Holsworth Wildlife Research Endowment (HWRE), and provided much information on arrow squid lifecycles. The University of Tasmania (UTAS), the Victorian Marine Science Consortium (VMSC) and the Department of Primary Industries — Fisheries Victoria, assisted in providing laboratories, desks and utilities, as well as offering a wonderful and inviting working environment. -

(Haliaeetus Leucogaster) and the Eastern Osprey (Pandion Cristatus
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 37 - PART 1 - March - 2011 Journal of The South Australian Ornithological Association Inc. In this issue: Osprey and White-bellied Sea-Eagle populations in South Australia Birds of Para Wirra Recreation Park Bird report 2009 March 2011 1 Distribution and status of White-bellied Sea-Eagle, Haliaeetus leucogaster, and Eastern Osprey, Pandion cristatus, populations in South Australia T. E. DENNIS, S. A. DETmAR, A. V. BROOkS AND H. m. DENNIS. Abstract Surveys throughout coastal regions and in the INTRODUCTION Riverland of South Australia over three breeding seasons between May 2008 and October 2010, Top-order predators, such as the White-bellied estimated the population of White-bellied Sea- Sea-Eagle, Haliaeetus leucogaster, and Eastern Eagle, Haliaeetus leucogaster, as 70 to 80 pairs Osprey, Pandion cristatus, are recognised and Eastern Osprey, Pandion cristatus, as 55 to indicator species by which to measure 65 pairs. Compared to former surveys these data wilderness quality and environmental integrity suggest a 21.7% decline in the White-bellied Sea- in a rapidly changing world (Newton 1979). In Eagle population and an 18.3% decline for Eastern South Australia (SA) both species have small Osprey over former mainland habitats. Most (79.2%) populations with evidence of recent declines sea-eagle territories were based on offshore islands linked to increasing human activity in coastal including Kangaroo Island, while most (60.3%) areas (Dennis 2004; Dennis et al. 2011 in press). osprey territories were on the mainland and near- A survey of the sea-eagle population in the shore islets or reefs. The majority of territories were mid 1990s found evidence for a decline in the in the west of the State and on Kangaroo Island, with breeding range since European colonisation three sub-regions identified as retaining significant (Dennis and Lashmar 1996). -

080058-89.02.017.Pdf
t9l .Ig6I pup spu?Fr rr"rl?r1mv qnos raq1oaqt dq panqs tou pus 916I uao^\teq sluauennboJ puu surelqord lusue8suuur 1eneds wq sauo8a uc .fu1mpw snorru,r aql uI luar&(oldua ',uq .(tg6l a;oJareqt puuls1oore8ueltr 0t dpo 1u reted lS sr ur saiuzqc aql s,roqs osIB elqeJ srqt usrmoJ 'urpilsny 'V'S) puels tseSrul geu oq; ur 1sa?re1p4ql aql 3o luetugedeq Z alq?J rrr rtr\oqs su padoldua puu prr"lsr aroqsJJorrprJpnsnv qlnos lsJErel aql ruJ ere,u eldoed ZS9 I feqf pa roqs snsseC srllsll?ls dq r ?olp sp qlr^\ puplsl oorp8ue1 Jo neomg u"rl?Jlsnv eql uo{ sorn8g luereJ lsour ,u1 0g€ t Jo eW T86I ul puu 00S € ,{lel?urxorddu sr uoqelndod '(derd ur '8ur,no:8 7r luosaJd eqJ petec pue uoqcnpord ,a uosurqoU) uoqecrJqndro3 peredard Smeq ,{11uermc '(tg6l )potsa^q roJ perualo Suraq puel$ oql Jo qJnu eru sda,r-rnsaseql3o EFSer pa[elop aqJ &usJ qlvrr pedolaaap fuouoce Surqsg puu Surure; e puu pue uosurqo;) pegoder useq aleq pesn spoporu palles-er su,rr prrqsr eql sreo,{ Eurpeet:ns eq1 re,ro prru s{nser druuruqord aqt pue (puep1 ooreSuqtr rnq 698I uI peuopwqs sE^\ elrs lrrrod seaeell eql SumnJcxa) sprrelsl aroqsJJo u"{e4snv qlnos '998I raqueJeo IIl eprelepv Jo tuaruslDesIeuroJ oqt aql Jo lsou uo palelduor ueeq A\ou aaeq s,{e,rrns aro3aq ,(ueduo3 rr"4u4snv qtnos qtgf 'oAE eqt dq ,{nt p:6o1org sree,{009 6 ol 000 L uea r1aq palulosr ur slors8rry1 u,no1paserd 6rll J?eu salaed 'o?e ;o lrrrod ererrirspuulsr Surura sr 3ql Jo dllJolpu aq; sree,{ paqslqplse peuuurad lE s?a\lueurep1os uuedorng y 009 0I spuplsl dpearg pue uosr"ed pup o8e srea,{ 'seruolocuorJ-"3s rel?l puB -
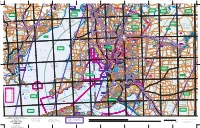
S P E N C E R G U L F S T G U L F V I N C E N T Adelaide
Yatala Harbour Paratoo Hill Turkey 1640 Sunset Hill Pekina Hill Mt Grainger Nackara Hill 1296 Katunga Booleroo "Avonlea" 2297 Depot Hill Creek 2133 Wilcherry Hill 975 Roopena 1844 Grampus Hill Anabama East Hut 1001 Dawson 1182 660 Mt Remarkable SOUTH Mount 2169 440 660 (salt) Mt Robert Grainger Scobie Hill "Mazar" vermin 3160 2264 "Manunda" Wirrigenda Hill Weednanna Hill Mt Whyalla Melrose Black Rock Goldfield 827 "Buckleboo" 893 729 Mambray Creek 2133 "Wyoming" salt (2658±) RANGE Pekina Wheal Bassett Mine 1001 765 Station Hill Creek Manunda 1073 proof 1477 Cooyerdoo Hill Maurice Hill 2566 Morowie Hill Nackara (abandoned) "Bulyninnie" "Oak Park" "Kimberley" "Wilcherry" LAKE "Budgeree" fence GILLES Booleroo Oratan Rock 417 Yeltanna Hill Centre Oodla "Hill Grange" Plain 1431 "Gilles Downs" Wirra Hillgrange 1073 B pipeline "Wattle Grove" O Tcharkuldu Hill T Fullerville "Tiverton 942 E HWY Outstation" N Backy Pt "Old Manunda" 276 E pumping station L substation Tregalana Baroota Yatina L Fitzgerald Bay A Middleback Murray Town 2097 water Ucolta "Pitcairn" E Buckleboo 1306 G 315 water AN Wild Dog Hill salt Tarcowie R Iron Peak "Terrananya" Cunyarie Moseley Nobs "Middleback" 1900 works (1900±) 1234 "Lilydale" H False Bay substation Yaninee I Stoney Hill O L PETERBOROUGH "Blue Hills" LC L HWY Point Lowly PEKINA A 378 S Iron Prince Mine Black Pt Lancelot RANGE (2294±) 1228 PU 499 Corrobinnie Hill 965 Iron Baron "Oakvale" Wudinna Hill 689 Cortlinye "Kimboo" Iron Baron Waite Hill "Loch Lilly" 857 "Pualco" pipeline Mt Nadjuri 499 Pinbong 1244 Iron -

Download Full Article 578.4KB .Pdf File
31 May 1988 Memoirs of the Museum of Victoria 49(1): 159-168 (1988) ISSN 0814-1827 https://doi.org/10.24199/j.mmv.1988.49.10 FIRST RECORDS OF NOTOTODARUS HAWAIIENSIS (BERRY, 1912) (CEPHALOPODA: OMMASTREPHIDAE) FROM NORTHERN AUSTRALIA WITH A RECONSIDERATION OF THE IDENTITY OF N. SLOANI PHILIPPINENS1S VOSS, 1962 By Malcolm Dunning Maritime Estate Management Branch, Queensland National Parks and Wildlife Service, PO Box 190, North Quay, Qld 4002, Australia Abstract Dunning, M., 1988. First records of Notoiociarus hawaiiensis (Berry, 1912) (Cephalopoda: Om- mastrephidae) from northern Australia with a reconsideration of the identity of N. sloani philip- pinensis Voss, 1962. Memoirs of the Museum of Victoria 49: 159-168. Nototodarus hawaiiensis (Berry, 1912) is reported for the first time from northern Australian continental slope waters and distribution and life history are discussed. Re-examination of the holotype of N. sloani philippinensis Voss, 1962 confirms that this subspecies is a junior syno- nym of N. hawaiiensis and that the paratype is referrable to Todarodes pacificus Steenstrup, 1880. Introduction (1985) tentatively assigned to this species specimens Recent exploratory trawling for deep-water taken on jigs at a seamount off the coast of Chile. 1973 was crustaceans in north-western and north-eastern N. nipponicus Okutani and Uemura, Australian continental slope waters yielded signifi- described from jig-caught specimens from southern characterised cant numbers of a large ommastrephid squid, as- Honshu, Japan. N. nipponicus was very broad fin relative to man- signed to the genus Nototodarus Pfeffer, 1912 on by "rough" skin, a angle. In a recent paper, the basis of the simple foveola in the funnel groove, tle length and large fin considered N.