Coarctation of the Aorta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
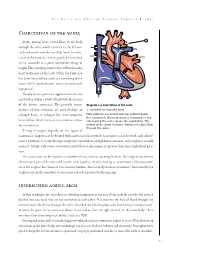
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery
Pediat. Res. 6: 693-700 (1972) Acetylcholine neonate atropine prematurity bradykinin sympathetic nervous system ductus arteriosus The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery INGRID OBERHANSLI-WEISS, MICHAEL A. HEYMANN1391, ABRAHAM M. RUDOLPH, AND KENNETH L. MELMON Cardiovascular Research Institute and Departments of Pediatrics, Physiology and Pharmacology, University of California San Francisco, San Francisco, California, USA Extract Response of the ductus arteriosus and umbilical artery to changes in oxygen tension, to acetylcholine, and to sympathetic and parasympathetic blocking agents was studied in vitro in isolated rings obtained from 22 fetal lambs of 98- to 147-day gestation. After stabilization of tension at a baseline level (0.3-0.7 g) in a PO2 environment of 35-45 mm Hg, both increase of the PO2 to 550 mm Hg and decrease of the PO2 to 8 mm Hg of the bathing solution produced constriction. The mean maximal tension developed by the ductus arteriosus.was 3.91 g at high PO2 and 3.87 g at low POr The increase in maximal tension developed with advancing gestation was also similar at both high and low POj. At P02 levels of 8-550 mm Hg, acetylcholine produced a further increase in tension, whereas bradykinin only produced an increase in tension at high PO2- Alpha and beta sympathetic blockade had no effect on the constrictor response to oxygen. Atropine relaxed the ductus arteriosus and umbilical artery at both high and low Po2 levels; the degree of relaxation was related to drug concentration. Acetylcholin- esterase also relaxed the ductus arteriosus constricted by oxygen. -

Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Acta Cardiol Sin 2016;32:731-737 Brief Report doi: 10.6515/ACS20160205A Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure Ali Yildirim,1 Alperen Aydin,2 Tevfik Demir,1 Fatma Aydin,2 Birsen Ucar1 and Zubeyir Kilic1 Background: The aim of the present study was to evaluate the echocardiographic follow-up of patent foramen ovale, which is considered a potential etiological factor in various diseases, and to determine the factors affecting spontaneous closure. Methods: Between January 2000 and June 2012, records of 918 patients with patent foramen ovale were retrospectively reviewed. Patency of less than 3 mm around the fossa ovalis is called patent foramen ovale. Patients with cyanotic congenital heart diseases, severe heart valve disorders and severe hemodynamic left to right shunts were excluded from the study. The patients were divided into three groups based on age; 1 day-1 monthingroup1,1month-12monthsingroup2,andmorethan12monthsingroup3. Results: Of the 918 patients, 564 (61.4%) had spontaneous closure, 328 (35.8%) had patent foramen ovale continued, 15 (1.6%) patients had patent foramen ovale enlarged to 3-5 mm, 6 patients were enlarged to 5-8 mm, and in one patient patent foramen ovale reached to more than 8 mm size. Defect was spontaneously closed in 65.9% of the patients in group 1, 66.7% of the patients in group 2, and 52.3% of the patients in group 3. There was a negative correlation between the age of diagnosis and spontaneous closure (p < 0.05). Gender, prematurity and coexisting malformations such as patent ductus arteriosus and atrial septal aneurysm did not have any effect on spontaneous closure of patent foramen ovale (p > 0.05). -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

[email protected]
Embryology)**@gmail.com 1-To serve prenatal needs. 2-To permit modifications at birth, which establish the neonatal circulation #Remember : Good respiration in the newborn infant is dependent upon normal circulatory changes at birth. Three structures are very important in the transitional circulation: 1- Ductus venosus. 2- Ductus arteriosus. 3- Foramen ovale Embryology)**@gmail.com Blood reaches & leaves the fetus through the umbilical cord and it Contains two arteries and one vein. 1- Highly oxygenated blood passes from the placenta through the umbilical vein. 2-Half of this blood reaches the IVC through the ductus venosus. 3- The other half passes to liver sinusoids then to the IVC. 4- Blood of the IVC reaches the right atrium, then left atrium through the Foramen Ovale (an opening between the two atrium). 5- Then to the left ventricle to the ascending aorta, and the aortic arch to supply head & neck brain, cardiac muscle and upper limbs with highly oxygenated blood. 6- Small amount of highly oxygenated blood in right atrium mixes with venous blood of the SVC passes to right ventricle. 7- Then to the pulmonary artery (lungs are not functioning yet) then to ductus arteriosus to the descending aorta, to lower half of fetal body. 8- Then back to placenta via the two umbilical arteries. Embryology)**@gmail.com 12 # After Ligation of the umbilical cord there will be Sudden fall of blood pressure in the IVC and the right Atrium Also the valve of the ductus venosus constricts. # After Aeration ( ventilation ) of the lungs at birth: 1- Marked increase in the pulmonary blood flow due to functioning of the lungs and increase pressure in left atrium causing physiological colsure. -

Aortic Arches
Human Embryology: Heart Development II Kimara L. Targoff, M.D. Division of Pediatric Cardiology, Columbia University Medical Center Developmental Genetics Program, Skirball Institute, NYU School of Medicine Human Vascular Development • Overview • Aortic Arch Development • Arterial Vascular Development • Venous System Development • Lymphatic Development • Transition from Fetal to Post-Natal Circulation Development of the Arterial and Venous Systems Cranial Ends of the Dorsal Aortae Form a Dorsoventral Loop: The First Aortic Arch Aortic Arches Arise in a Craniocaudal Sequence Surrounding the Pharynx Aortic Arches Give Rise to Important Head, Neck, and Upper Thorax Vessels Aortic Arch Development in the Chick Embryo Fgf8 is Required for Pharyngeal Arch Development in Mouse Abu-Issa, R. et al., Development 2002. Cardiovascular and Thymic Defects in Tbx1 Hypomorphic Mutant Neonates Hu, T. et al., Development 2004. Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg 6 Aortic sac Truncus arteriosus Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 7 iseg 4 7 iseg 6 Harsh Thaker Aortic Arch and Derivatives RCC LCC RSC LSC BCA DA Harsh Thaker Recurrent Laryngeal Nerves RCC LCC RSC LSC BCA DA Harsh Thaker Defects in Normal Regression of the Arterial System Lead to Vascular Anomalies • Double Aortic Arch – Failure of the -

Hypertension of the Pulmonary Circulation Due to Congenital
HIYPERTENSION OF THE PULMONARY CIRCULATION DUE TO CONGENITAL GLOMOID OBSTRUCTION OF THE PULMONARY ARTERIES EL MoscHcownz, M.D.; E. RunBiN, M.D,* AND L. SmAuss, M.D. From the Department of Pathology, The Mount Sinai Hospital, New York, N.Y. Our purpose is to report a hitherto unrecognized cause of hypertension of the pulmonary circulation. Although the histologic structure of the lesion has been frequently described, there has been a wide divergence in the interpretation of the pathogenesis and significance of the lesions. With a single exception,' the lesion has been viewed as the result and not the cause of hypertension in the pulmonary circulation. The proba- bility is strong, now that more attention may be paid to it, that the lesion will be reported more frequently. The fact that the 4 cases we have de- scribed were encountered within the space of a few years supports this surmise. CASE REPORTS Case I A 7-month-old boy entered the hospital with bronchopneumonia and congestive failure. The child suddenly developed respiratory failure and died. Necropsy Observations. The fifth fingers were shorter than normal, and there was an unusually wide separation between the first and second toes. The heart weighed 55 gmi (normal, 35.5 gin.). The right atrium was enlarged and lined by thickened endocardium. The foramen ovale was patent and bridged by a narrow fibrous band. The atrial septum was short, and a large defect was present. This constituted a com- bined ostium primum and a ventricular septal defect; it measured 2 cm. in diameter. As a result, a common atrioventricular canal with wide communication connected all chambers of the heart. -

Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure
International Journal of Molecular Sciences Review Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure Yu-Chi Hung 1,2, Jwu-Lai Yeh 1,3,4,5 ID and Jong-Hau Hsu 1,3,6,7,* 1 Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan; [email protected] (Y.-C.H.); [email protected] (J.-L.Y.) 2 Department of Pediatrics, St. Joseph Hospital, Kaohsiung 807, Taiwan 3 Department of Pharmacology, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan 4 Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung 807, Taiwan 5 Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 804, Taiwan 6 Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan 7 Department of Pediatrics, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan * Correspondence: [email protected]; Tel.: +886-7-3121101 (ext. 6507) Received: 4 May 2018; Accepted: 20 June 2018; Published: 25 June 2018 Abstract: The ductus arteriosus (DA) connects the main pulmonary artery and the aorta in fetal circulation and closes spontaneously within days after birth in normal infants. Abnormal patent DA (PDA) causes morbidities and mortality, especially in preterm infants. Closure of the DA is a complex interactive process involving two events: functional and anatomic closure. Functional closure by smooth muscle contraction was achieved through the regulatory factors of vaso-reactivity. These factors include oxygen sensing system, glutamate, osmolality, prostaglandin E2, nitric oxide, and carbon monoxide. Anatomic closure by vascular remodeling involved several vascular components including endothelium, extracellular matrix, smooth muscle cells, and intraluminal blood cells.