Thoracic Aorta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiac CT - Quantitative Evaluation of Coronary Calcification
Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Imaging of the Heart Effective Date: January 1, 2018 Proprietary Date of Origin: 03/30/2005 Last revised: 11/14/2017 Last reviewed: 11/14/2017 8600 W Bryn Mawr Avenue South Tower - Suite 800 Chicago, IL 60631 P. 773.864.4600 Copyright © 2018. AIM Specialty Health. All Rights Reserved www.aimspecialtyhealth.com Table of Contents Description and Application of the Guidelines ........................................................................3 Administrative Guidelines ........................................................................................................4 Ordering of Multiple Studies ...................................................................................................................................4 Pre-test Requirements ...........................................................................................................................................5 Cardiac Imaging ........................................................................................................................6 Myocardial Perfusion Imaging ................................................................................................................................6 Cardiac Blood Pool Imaging .................................................................................................................................12 Infarct Imaging .....................................................................................................................................................15 -
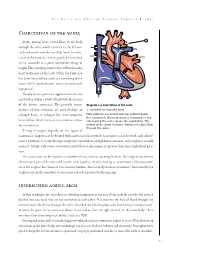
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Of the Pediatric Mediastinum
MRI of the Pediatric Mediastinum Dianna M. E. Bardo, MD Director of Body MR & Co-Director of the 3D Innovation Lab Disclosures Consultant & Speakers Bureau – honoraria Koninklijke Philips Healthcare N V Author – royalties Thieme Publishing Springer Publishing Mediastinum - Anatomy Superior Mediastinum thoracic inlet to thoracic plane thoracic plane to diaphragm Inferior Mediastinum lateral – pleural surface anterior – sternum posterior – vertebral bodies Mediastinum - Anatomy Anterior T4 Mediastinum pericardium to sternum Middle Mediastinum pericardial sac Posterior Mediastinum vertebral bodies to pericardium lateral – pleural surface superior – thoracic inlet inferior - diaphragm Mediastinum – MR Challenges Motion Cardiac ECG – gating/triggering Breathing Respiratory navigation Artifacts Intubation – LMA Surgical / Interventional materials Mediastinum – MR Sequences ECG gated/triggered sequences SSFP – black blood SE – IR – GRE Non- ECG gated/triggered sequences mDIXON (W, F, IP, OP), eTHRIVE, turbo SE, STIR, DWI Respiratory – triggered, radially acquired T2W MultiVane, BLADE, PROPELLER Mediastinum – MR Sequences MRA / MRV REACT – non Gd enhanced Gd enhanced sequences THRIVE, mDIXON, mDIXON XD Mediastinum – Contents Superior Mediastinum PVT Left BATTLE: Phrenic nerve Vagus nerve Structures at the level of the sternal angle Thoracic duct Left recurrent laryngeal nerve (not the right) CLAPTRAP Brachiocephalic veins Cardiac plexus Aortic arch (and its 3 branches) Ligamentum arteriosum Thymus Aortic arch (inner concavity) Trachea Pulmonary -

Comparison of Echocardiography and Angiography in Determining the Cause of Severe Aortic Regurgitation
Br Heart J: first published as 10.1136/hrt.51.1.36 on 1 January 1984. Downloaded from Br Heart J 1984; 51: 36-45 Comparison of echocardiography and angiography in determining the cause of severe aortic regurgitation NICHOLAS L DEPACE, PASQUALE F NESTICO, MORRIS N KOTLER, GARY S MINTZ, DEMETRIOS KIMBIRIS, INDER P GOEL, E ELAINE GLAZIER-LASKEY, JOHN ROSS From the LikoffCardiovascular Institute, Hahnemann University, Philadelphia, Pennsylvania, USA SUMMARY To assess the accuracy of echocardiography in determining the cause of aortic regurgita- tion M mode and cross sectional echocardiography were compared with angiography in 43 patients with predominant aortic regurgitation. Each patient had all three investigations performed during the same admission to hospital. In each instance, the cause of aortic regurgitation was confirmed at surgery or necropsy. Seventeen patients had rheumatic aortic valve disease, 13 bacterial endocarditis with a perforated or partially destroyed cusp, five a biscuspid aortic valve (four with a history of endocarditis), and eight aortic regurgitation secondary to aortic root dilatation or aneurysm. Overall sensitivity of echocardiography and aortography was 84% in determining the cause of aortic regurgi- tation. Thus, rheumatic valve disease and endocarditis appear to be the most common causes of severe aortic regurgitation in this hospital based population. Furthermore, echocardiography is a sensitive non-invasive technique for determining the cause of aortic regurgitation and allows differentiation of valvular from root causes of aortic regurgitation. Aortic regurgitation may be caused by valvular dis- ment for predominant aortic regurgitation were http://heart.bmj.com/ ease, aortic root disease, or a combination of both. reviewed. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Fetal Descending Aorta/Umbilical Artery Flow Velocity Ratio in Normal Pregnancy at 36-40 Weeks of Gestational Age Riyadh W Alessawi1
American Journal of BioMedicine AJBM 2015; 3(10):674 - 685 doi:10.18081/2333-5106/015-10/674-685 Fetal descending aorta/umbilical artery flow velocity ratio in normal pregnancy at 36-40 Weeks of gestational age Riyadh W Alessawi1 Abstract Doppler velocimetry studies of placental and aortic circulation have gained a wide popularity as it can provide important information regarding fetal well-being and could be used to identify fetuses at risk of morbidity and mortality, thus providing an opportunity to improve fetal outcomes. Prospective longitudinal study conducted through the period from September 2011–July 2012, 125 women with normal pregnancy and uncomplicated fetal outcomes were recruited and subjected to Doppler velocimetry at different gestational ages, from 36 to 40 weeks. Of those, 15 women did not fulfill the protocol inclusion criteria and were not included. In the remaining 110 participants a follow up study of Fetal Doppler velocimetry of Ao and UA was performed at 36 – 40 weeks of gestation. Ao/UA RI: 1.48±0.26, 1.33±0.25, 1.37± 0.20, 1.28±0.07 and 1.39±0.45 respectively and the 95% confidence interval of the mean for five weeks 1.13-1.63. Ao/UA PI: 2.83±2.6, 1.94±0.82, 2.08±0.53, 1.81± 0.12 and 3.28±2.24 respectively. Ao/UA S/D: 2.14±0.72, 2.15±1.14, 1.75±0.61, 2.52±0.18 and 2.26±0.95. The data concluded that a nomogram of descending aorto-placental ratio Ao/UA, S/D, PI and RI of Iraqi obstetric population was established. -

“Cardiac Solution” Program Tip Sheet
“Cardiac Solution” Program Tip Sheet MYOCARDIAL PERFUSION IMAGING (MPI) vs. STRESS ECHOCARDIOGRAPHY (SE) Main Points about the Two Tests: Both tests have equal diagnostic accuracy for coronary artery disease, with MPI showing greater sensitivity and SE showing greater specificity. MPI is based upon the expectation of relatively reduced blood flow in a myocardial segment during exercise or pharmacologic coronary microvessel dilation, while SE is based upon development of wall motion abnormality provoked by myocardial ischemia during treadmill exercise or similar stress. In order to perform a SE, one would prefer to have a patient who could perform treadmill exercise well, along with a good acoustic imaging window, while MPI can be performed with either exercise or the pharmacologic option. Exercise can also provide the additional information from the EKG, when the baseline EKG does not already have substantial abnormality (e.g. a 1 mm ST segment depression at baseline, left bundle branch block, ventricular pacing, PVCs, or pre-excitation). Even with MPI, an exercise modality is preferred over pharmacologic vasodilation due to the additional functional and EKG information inherent in exercise testing. However, in some patients, such as those with a pre-existing wall motion abnormality, left bundle branch block, ventricular paced rhythms, frequent PVCs, or pre-excitation (WPW), the related cardiac contraction pattern during exercise could obscure the effects of ischemia, making a pharmacologic approach more helpful. The radiation exposure of SE is zero, while MPI incurs a radiation Radiation Exposure dose of 7-24 mSv (the equivalent of about 117-400 PA & lateral chest X-rays), with an increase in lifetime radiation exposure and its MPI: 7 - 24 mSv associated cancer risk. -

The Descending Thoracic Aorta Morphological Characteristics
ARS Medica Tomitana - 2016; 3(22): 186 - 191 10.1515/arsm-2016-0031 Malik S., Bordei P., Rusali A., Iliescu D. M. The descending thoracic aorta morphological characteristics Faculty of Medicine, “Ovidius” University, Constanta ABSTRACT Introduction Our study was conducted by consulting angioCT sites made on a CT GE LightSpeed VCT64 Slice CT and a CT GE LightSpeed 16 Slice CT, following the path and relationships of the descending thoracic aorta against the vertebral column, outside diameters thereof at the Descending thoracic aorta extends from the thoracic vertebrae T4, T7, T12 and posterior intercostal aortic arch (which it continues) and aortic hiatus of arteries characteristics. The origin of of the descending the diaphragm at level of T12 vertebra [1,2,3,4,5] thoracic aorta we found most commonly on the left corresponding to the front of T10 [6], level that flank of the lower edge of the vertebral body T4, but continues with the abdominal descending aorta. She I have encountered cases where it had come above the enters the posterior mediastinum at the T4 vertebra and lower edge of T4 on level of intervertebral disc T4-T5 or describes a trajectory which is vertically downward as even at the upper edge of T5 vertebral body. At thoracic a whole, being slightly inferior oblique and to the right, vertebra T4, on a total of 30 cases, the descending thoracic aorta present a diameter of 20.0 to 32.6 mm, then, first at a distance of 2-3 cm midline, progressive values that correspond to male gender and to females approach to become median and prevertebral at the diameter ranging from 25.5 to 27, 4 mm. -

Inferior Phrenic Artery, Variations in Origin and Clinical Implications – a Case Study
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) E-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 7, Issue 6 (Mar.- Apr. 2013), PP 46-48 www.iosrjournals.org Inferior Phrenic Artery, Variations in Origin and Clinical Implications – A Case Study 1 2 3 Dr.Anupama D, Dr.R.Lakshmi Prabha Subhash .Dr. B.S Suresh Assistant Professor. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Professor & HOD. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Associate professor.Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Abstract:Variations in the branching pattern of abdominal aorta are quite common, knowledge of which is required to avoid complications during surgical interventions involving the posterior abdominal wall. Inferior Phrenic Arteries, the lateral aortic branches usually arise from Abdominal Aorta ,just above the level of celiac trunk. Occasionally they arise from a common aortic origin with celiac trunk, or from the celiac trunk itself or from the renal artery. This study describes the anomalous origin of this lateral or para aortic branches in the light of embryological and surgical basis. Knowledge of such variations has important clinical significance in abdominal operations like renal transplantation, laparoscopic surgery, and radiological procedures in the upper abdomen or invasive arterial procedures . Keywords: Abdominal Aorta, Celiac Trunk(Ct), Diaphragm, Inferior Phrenic Artery (Ipa), Retro Peritoneal, Renal Artery(Ra). I. Introduction The abdominal aorta begins from the level of 12th thoracic vertebra after passing through the Osseo aponeurotic hiatus of diaphragm. It courses downwards with Inferior vena cava to its right and terminates at the level of 4th lumbar vertebra by dividing in to two terminal branches. -

DIAGNOSTIC SERVICES Diagnostic Imaging
DIAGNOSTIC SERVICES Diagnostic Imaging Diagnostic services are a critical Day Kimball Healthcare provides high quality, convenient diagnostic component in the continuum of care. imaging services at four locations across northeast Connecticut. Our state-of-the-art facilities use the latest technologies. Our associated That’s why our diagnostic imaging and board-certified radiologists from Jefferson Radiology are experts in laboratory services utilize the latest the fields of diagnostic imaging and interventional radiology. And, technologies to provide you with the our integrated Electronic Medical Records (EMR) system means your highest quality testing and most efficient care team will have access to your imaging results as soon as they’re results reporting possible. available, providing you with a shorter wait time to receive results and Multiple locations across northeast helping you to avoid redundant testing so you can better control your Connecticut offer convenient access to a healthcare costs. full range of the most advanced testing Our services include: possible, while our electronic medical • CT (Computerized Tomography) Scans - Head and body scans, CT records system ensures that your results angiography, and CT-guided biopsies and surgical procedures. are instantly shared with your care team • DEXA (Bone Density/Bone Densiometry) Testing - We offer dual as soon as they’re available. energy bone density imaging for the detection of osteoporosis and Our certified and dedicated diagnostics high definition instant vertebral assessment for detection of spine professionals, accredited facilities, and fractures with rapid, low dose, single energy images in seconds. integrated network ensure that you’ll • Interventional Radiology - Image guided interventional services receive the highest quality, most efficient include a wide range of procedures, biopsies and pain management and reliable testing available, in a treatment. -

Surgical Indications. a Critical Point of View
Surgical indications in ascending aorta aneurysms: What do we know? Jean-Luc MONIN, MD, PhD. Institut Mutualiste Montsouris, Paris, FRANCE Disclosures related to this talk : www.imm.fr NONE 2 Clinical case www.imm.fr • A 40 year-old woman • Height: 172 cm/ Weight: 70 kg • Ascending aortic aneurysm • She wants to become pregnant (3-year old kid) • No Marfan syndrome or bicuspid AV • Younger sister: sudden unexplained death (at 18 years old) • 2012 (MRI): Valsalva: 42 mm, tubular: 43 mm • 2013 (MRI): Valsalva: 40 mm, tubular: 45 mm 3 www.imm.fr www.imm.fr www.imm.fr www.imm.fr www.imm.fr www.imm.fr What would we advise to this woman www.imm.fr ? A. Pregnancy is temporarily contra indicated, MRI or cardiac CT is needed B. No contra indication for pregnancy, TTE or MRI at 1 year 10 Thoracic aortic aneurysm www.imm.fr Thoracic Aortic Aneurysm: An indolent but virulent process www.imm.fr Ascending Aorta 1 mm/ year 45 mm 60 mm 3 mm/ year 34% Rate of aortic dilatation is EXPONENTIAL • Expansion rate ≈ 2.1 mm/ year for an initial diameter of 35-40 mm (%) rupture dissection/ of risk Lifetime Aortic diameter (cm) • Expansion rate ≈ 5.6 mm/ year for aneurysms ≥ 60 mm Coady et al. J Thorac Cardiovasc Surg. 1997;113: 476 Mechanical properties of human ascending aorta : >6 cm is the limit www.imm.fr Exponential relationship between wall stress and aneurysm size in ascending aortic aneurysms. • Pink columns : SBP =100 mm Hg • Purple columns : SBP = 200 mm Hg • Range of maximum tensile strength of the human aorta : 800 to 1,000 kPa Koullias et al.