The Aorta, and Pulmonary Blood Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Artery Visualizations for Heart Disease Diagnosis
Evaluation of Artery Visualizations for Heart Disease Diagnosis Michelle A. Borkin, Student Member, IEEE, Krzysztof Z. Gajos, Amanda Peters, Dimitrios Mitsouras, Simone Melchionna, Frank J. Rybicki, Charles L. Feldman, and Hanspeter Pfister, Senior Member, IEEE Fig. 1. Left: Traditional 2D projection (A) of a single artery, and 3D representation (C) of a right coronary artery tree with a rainbow color map. Right: 2D tree diagram representation (B) and equivalent 3D representation (D) of a left coronary artery tree with a diverging color map. Abstract—Heart disease is the number one killer in the United States, and finding indicators of the disease at an early stage is critical for treatment and prevention. In this paper we evaluate visualization techniques that enable the diagnosis of coronary artery disease. A key physical quantity of medical interest is endothelial shear stress (ESS). Low ESS has been associated with sites of lesion formation and rapid progression of disease in the coronary arteries. Having effective visualizations of a patient’s ESS data is vital for the quick and thorough non-invasive evaluation by a cardiologist. We present a task taxonomy for hemodynamics based on a formative user study with domain experts. Based on the results of this study we developed HemoVis, an interactive visualization application for heart disease diagnosis that uses a novel 2D tree diagram representation of coronary artery trees. We present the results of a formal quantitative user study with domain experts that evaluates the effect of 2D versus 3D artery representations and of color maps on identifying regions of low ESS. We show statistically significant results demonstrating that our 2D visualizations are more accurate and efficient than 3D representations, and that a perceptually appropriate color map leads to fewer diagnostic mistakes than a rainbow color map. -

Prep for Practical II
Images for Practical II BSC 2086L "Endocrine" A A B C A. Hypothalamus B. Pineal Gland (Body) C. Pituitary Gland "Endocrine" 1.Thyroid 2.Adrenal Gland 3.Pancreas "The Pancreas" "The Adrenal Glands" "The Ovary" "The Testes" Erythrocyte Neutrophil Eosinophil Basophil Lymphocyte Monocyte Platelet Figure 29-3 Photomicrograph of a human blood smear stained with Wright’s stain (765). Eosinophil Lymphocyte Monocyte Platelets Neutrophils Erythrocytes "Blood Typing" "Heart Coronal" 1.Right Atrium 3 4 2.Superior Vena Cava 5 2 3.Aortic Arch 6 4.Pulmonary Trunk 1 5.Left Atrium 12 9 6.Bicuspid Valve 10 7.Interventricular Septum 11 8.Apex of The Heart 9. Chordae tendineae 10.Papillary Muscle 7 11.Tricuspid Valve 12. Fossa Ovalis "Heart Coronal Section" Coronal Section of the Heart to show valves 1. Bicuspid 2. Pulmonary Semilunar 3. Tricuspid 4. Aortic Semilunar 5. Left Ventricle 6. Right Ventricle "Heart Coronal" 1.Pulmonary trunk 2.Right Atrium 3.Tricuspid Valve 4.Pulmonary Semilunar Valve 5.Myocardium 6.Interventricular Septum 7.Trabeculae Carneae 8.Papillary Muscle 9.Chordae Tendineae 10.Bicuspid Valve "Heart Anterior" 1. Brachiocephalic Artery 2. Left Common Carotid Artery 3. Ligamentum Arteriosum 4. Left Coronary Artery 5. Circumflex Artery 6. Great Cardiac Vein 7. Myocardium 8. Apex of The Heart 9. Pericardium (Visceral) 10. Right Coronary Artery 11. Auricle of Right Atrium 12. Pulmonary Trunk 13. Superior Vena Cava 14. Aortic Arch 15. Brachiocephalic vein "Heart Posterolateral" 1. Left Brachiocephalic vein 2. Right Brachiocephalic vein 3. Brachiocephalic Artery 4. Left Common Carotid Artery 5. Left Subclavian Artery 6. Aortic Arch 7. -
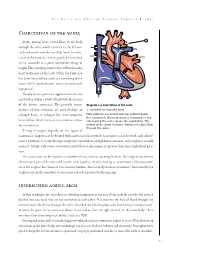
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Vascular Anomalies Compressing the Oesophagus and Trachea
Thorax: first published as 10.1136/thx.24.3.295 on 1 May 1969. Downloaded from T7horax (1969), 24, 295. Vascular anomalies compressing the oesophagus and trachea J. C. R. LINCOLN, P. B. DEVERALL, J. STARK, E. ABERDEEN, AND D. J. WATERSTON From the Hospital for Sick Children, Great Ormond Street, London W.C.I Vascular rings formed by anomalies of major arteries can compress the trachea and oesophagus so much as to cause respiratory distress and dysphagia. Twenty-nine patients with this condition are reviewed and discussed in five groups. The symptoms and signs are noted. Radiological examination by barium swallow is the most useful diagnostic aid. Symptoms can only be relieved by operation. The trachea is often deformed at the site of the constricting ring. Only infrequently is there immediate relief from the pre-operative symptoms. Two babies were successfully treated for an aberrant left pulmonary artery. The diagnosis and treatment of major arterial DOUBLE AORTIC ARCH Nineteen children were anomalies which cause compression of the oeso- treated for some form of double aortic arch. Their phagus and trachea are now well established. age at operation ranged from 1 week to 11 months, Although an aberrant right subclavian artery was but the majority were treated at about the age of described in 1794 by Bayford (Fig. 1), who gave 5 to 6 months (Fig. 2). Frequently the presenting the detailed post-mortem finding in a woman who symptoms had been noticed since birth but for had died from starvation secondary to this varying reasons there was delay in making the http://thorax.bmj.com/ anomaly, and a double aortic arch was described correct diagnosis. -

Fetal Descending Aorta/Umbilical Artery Flow Velocity Ratio in Normal Pregnancy at 36-40 Weeks of Gestational Age Riyadh W Alessawi1
American Journal of BioMedicine AJBM 2015; 3(10):674 - 685 doi:10.18081/2333-5106/015-10/674-685 Fetal descending aorta/umbilical artery flow velocity ratio in normal pregnancy at 36-40 Weeks of gestational age Riyadh W Alessawi1 Abstract Doppler velocimetry studies of placental and aortic circulation have gained a wide popularity as it can provide important information regarding fetal well-being and could be used to identify fetuses at risk of morbidity and mortality, thus providing an opportunity to improve fetal outcomes. Prospective longitudinal study conducted through the period from September 2011–July 2012, 125 women with normal pregnancy and uncomplicated fetal outcomes were recruited and subjected to Doppler velocimetry at different gestational ages, from 36 to 40 weeks. Of those, 15 women did not fulfill the protocol inclusion criteria and were not included. In the remaining 110 participants a follow up study of Fetal Doppler velocimetry of Ao and UA was performed at 36 – 40 weeks of gestation. Ao/UA RI: 1.48±0.26, 1.33±0.25, 1.37± 0.20, 1.28±0.07 and 1.39±0.45 respectively and the 95% confidence interval of the mean for five weeks 1.13-1.63. Ao/UA PI: 2.83±2.6, 1.94±0.82, 2.08±0.53, 1.81± 0.12 and 3.28±2.24 respectively. Ao/UA S/D: 2.14±0.72, 2.15±1.14, 1.75±0.61, 2.52±0.18 and 2.26±0.95. The data concluded that a nomogram of descending aorto-placental ratio Ao/UA, S/D, PI and RI of Iraqi obstetric population was established. -

In the Pulmonary Arteries, Capillaries, and Veins
Longitudinal Distribution of Vascular Resistance in the Pulmonary Arteries, Capillaries, and Veins JEROME S. BRODY, EDWARD J. STEMMLER, and ARTHu B. DuBois From the Department of Physiology, Division of Graduate Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104 A B S T R A C T A new method has been described monary artery pressure averaged 20.4 cm H2O, for measuring the pressure and resistance to blood and pulmonary vein pressure averaged 9.2 cm flow in the pulmonary arteries, capillaries, and H2O. These techniques also provide a way of ana- veins. Studies were performed in dog isolated lyzing arterial, capillary, and venous responses to lung lobes perfused at constant flow with blood various pharmacologic and physiologic stimuli. from a donor dog. Pulmonary artery and vein volume and total lobar blood volume were mea- INTRODUCTION sured by the ether plethysmograph and dye- dilution techniques. The longitudinal distribution The arterioles are the major resistance vessels in of vascular resistance was determined by analyzing the systemic vascular bed (1, 2), but it is uncer- the decrease in perfusion pressure caused by a tain whether the greatest resistance to blood flow bolus of low viscosity liquid introduced into the in the lungs is in the arteries, capillaries, or veins vascular inflow of the lobe. (1, 3, 4). The pulmonary arteries were responsible for Piiper (5), in 1958, reasoned from Poiseuille's 46% of total lobar vascular resistance, whereas law that, during constant perfusion, injection into the pulmonary capillaries and veins accounted for the bloodstream of a bolus of fluid with viscosity 34 and 20% of total lobar vascular resistance different from that of blood would cause a per- respectively. -

The Descending Thoracic Aorta Morphological Characteristics
ARS Medica Tomitana - 2016; 3(22): 186 - 191 10.1515/arsm-2016-0031 Malik S., Bordei P., Rusali A., Iliescu D. M. The descending thoracic aorta morphological characteristics Faculty of Medicine, “Ovidius” University, Constanta ABSTRACT Introduction Our study was conducted by consulting angioCT sites made on a CT GE LightSpeed VCT64 Slice CT and a CT GE LightSpeed 16 Slice CT, following the path and relationships of the descending thoracic aorta against the vertebral column, outside diameters thereof at the Descending thoracic aorta extends from the thoracic vertebrae T4, T7, T12 and posterior intercostal aortic arch (which it continues) and aortic hiatus of arteries characteristics. The origin of of the descending the diaphragm at level of T12 vertebra [1,2,3,4,5] thoracic aorta we found most commonly on the left corresponding to the front of T10 [6], level that flank of the lower edge of the vertebral body T4, but continues with the abdominal descending aorta. She I have encountered cases where it had come above the enters the posterior mediastinum at the T4 vertebra and lower edge of T4 on level of intervertebral disc T4-T5 or describes a trajectory which is vertically downward as even at the upper edge of T5 vertebral body. At thoracic a whole, being slightly inferior oblique and to the right, vertebra T4, on a total of 30 cases, the descending thoracic aorta present a diameter of 20.0 to 32.6 mm, then, first at a distance of 2-3 cm midline, progressive values that correspond to male gender and to females approach to become median and prevertebral at the diameter ranging from 25.5 to 27, 4 mm. -

Artery/Vein Classification of Blood Vessel Tree in Retinal Imaging
Artery/vein Classification of Blood Vessel Tree in Retinal Imaging Joaquim de Moura1, Jorge Novo1, Marcos Ortega1, Noelia Barreira1 and Pablo Charlon´ 2 1Departamento de Computacion,´ Universidade da Coruna,˜ A Coruna,˜ Spain 2Instituto Oftalmologico´ Victoria de Rojas, A Coruna,˜ Spain joaquim.demoura, jnovo, mortega, nbarreira @udc.es, [email protected] { } Keywords: Retinal Imaging, Vascular Tree, Segmentation, Artery/vein Classification. Abstract: Alterations in the retinal microcirculation are signs of relevant diseases such as hypertension, arteriosclerosis, or diabetes. Specifically, arterial constriction and narrowing were associated with early stages of hypertension. Moreover, retinal vasculature abnormalities may be useful indicators for cerebrovascular and cardiovascular diseases. The Arterio-Venous Ratio (AVR), that measures the relation between arteries and veins, is one of the most referenced ways of quantifying the changes in the retinal vessel tree. Since these alterations affect differently arteries and veins, a precise characterization of both types of vessels is a key issue in the development of automatic diagnosis systems. In this work, we propose a methodology for the automatic vessel classification between arteries and veins in eye fundus images. The proposal was tested and validated with 19 near-infrared reflectance retinographies. The methodology provided satisfactory results, in a complex domain as is the retinal vessel tree identification and classification. 1 INTRODUCTION Hence, direct analysis of many injuries caused by oc- ular pathologies can be achieved, as is the case, for The analysis of the eye fundus offers useful infor- example, the diabetic retinopathy (DR). The DR is a mation about the status of the different structures the diabetes mellitus complication, one of the principal human visual system integrates, as happens with the causes of blindness in the world (Pascolini, 2011). -

Inferior Phrenic Artery, Variations in Origin and Clinical Implications – a Case Study
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) E-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 7, Issue 6 (Mar.- Apr. 2013), PP 46-48 www.iosrjournals.org Inferior Phrenic Artery, Variations in Origin and Clinical Implications – A Case Study 1 2 3 Dr.Anupama D, Dr.R.Lakshmi Prabha Subhash .Dr. B.S Suresh Assistant Professor. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Professor & HOD. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Associate professor.Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Abstract:Variations in the branching pattern of abdominal aorta are quite common, knowledge of which is required to avoid complications during surgical interventions involving the posterior abdominal wall. Inferior Phrenic Arteries, the lateral aortic branches usually arise from Abdominal Aorta ,just above the level of celiac trunk. Occasionally they arise from a common aortic origin with celiac trunk, or from the celiac trunk itself or from the renal artery. This study describes the anomalous origin of this lateral or para aortic branches in the light of embryological and surgical basis. Knowledge of such variations has important clinical significance in abdominal operations like renal transplantation, laparoscopic surgery, and radiological procedures in the upper abdomen or invasive arterial procedures . Keywords: Abdominal Aorta, Celiac Trunk(Ct), Diaphragm, Inferior Phrenic Artery (Ipa), Retro Peritoneal, Renal Artery(Ra). I. Introduction The abdominal aorta begins from the level of 12th thoracic vertebra after passing through the Osseo aponeurotic hiatus of diaphragm. It courses downwards with Inferior vena cava to its right and terminates at the level of 4th lumbar vertebra by dividing in to two terminal branches.